Abstract
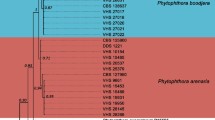
Hendersonia osteospermi was found for the first time in Australia on leaf spots of the introduced invasive plant Chrysanthemoides monilifera ssp. rotundata (bitou bush) in coastal regions of New South Wales. Pathogenicity tests on species from 11 tribes in the family Asteraceae, demonstrated that H. osteospermi caused severe necrosis on leaves and stems of C. monilifera ssp. rotundata and its congener C. monilifera ssp. monilifera (boneseed). Small necrotic spots also developed on Osteospermum fruticosum and Dimorphotheca cuneata in the Calenduleae and on Helianthus annuus (sunflower) in the Heliantheae. None of the other plant species tested developed leaf spots, although H. osteospermi was re-isolated from senescent leaves of Cynara scolymus (globe artichoke) in the Cynareae and Vernonia cinerea in the Vernonieae. Single ascospores from ascomata of a Pleospora-like fungus found on diseased stems of bitou bush produced H. osteospermi in culture, which proved the anamorph/teleomorph connection. The ITS region of both a single-ascospore isolate and a single-conidium isolate were sequenced and found to be identical. The taxonomic status of H. osteospermi is re-examined and Austropleospora osteospermi gen. et sp. nov. is described as its teleomorph based on morphology, host range tests and DNA sequence analysis. The potential of A. osteospermi for the biological control of bitou bush and boneseed in Australia is discussed.



Similar content being viewed by others
References
Barr DA (1965) Restoration of coastal dunes after beach mining. Journal of the Soil Conservation Service of New South Wales 21:199–209
Brennan RM, Fitt BDL, Taylor GS, Colhoun J (1985) Dispersal of Septoria nodorum pycnidiospores by simulated rain and wind. Phytopathol Z 112:291–297
Briese DT (2004) Weed biological control: applying science to solve seemingly intractable problems. Aust J Entomol 42:304–317
Brown JKM, Hovmøller MS (2002) Aerial dispersal of pathogens on the global and continental scales and its impact on plant disease. Science 297:537–541
Cother EJ (2000) Pathogenicity of Sclerotinia sclerotiorum to Chrysanthemoides monilifera ssp rotundata (Bitou bush) and selected species of the coastal flora in eastern Australia. Biol Control 18:10–17
Cother EJ, Nikandrow A, Gilbert RL (1996) Sclerotinia sclerotiorum, a potential biocontrol agent for Chrysanthemoides monilifera (bitoubush). In: Moran VC, Hoffmann JH (eds) Proceedings of the IX International Symposium on Biological Control of Weeds. University of Cape Town, South Africa, pp 529–530
Crous PW, Groenewald JZ (2006) Camarosporium mamanes Crous, sp. nov. Fungal Planet 5
Crous PW, Phillips AJL, Baxter AP (2000) Phytopathogenic Fungi from South Africa. Department of Plant Pathology Press, University of Stellenbosch
Doidge EM (1950) The South African fungi and lichens to the end of 1945. Bothalia 5:1–1094
Downey PO, Holtkamp RH, Ireson JE, Kwong RM, Swirepik AE (2007) A review of the Chrysanthemoides monilifera biological control program in Australia: 1987–2005. Plant Prot Q 22:24–32
Evans HC, Fröhlich J, Shamoun SF (2001) Biological control of weeds. Fungal biocontrol agents of weeds. In: Pointing SB, Hyde KD (eds) Bio-exploitation of filamentous fungi. Hong Kong, Fungal Diversity Press, pp 349–401
French K, Ens E, Gosper CR, Lindsay E, Mason T, Owers B, Sullivan N (2008) Management implications of recent research into the effect of Bitou bush invasion. Plant Prot Q 23:24–28
Gardes A, Bruns TD (1993) ITS primers with enhanced specificity for basdiomycetes—application to the identification of mycorrhizae and rusts. Mol Ecol 2:113–118
Gorter GJMA (1981) Index of plant pathogens (II) and the diseases they cause in wild growing plants in South Africa. Republic of South Africa, Department of Agriculture and Fisheries, Science Bulletin No. 398
Hanlin RT (1990) Illustrated genera of ascomycetes. APS, St. Paul
Higgins D, Thompson J, Gibson T, Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Index Fungorum (2008) http://www.speciesfungorum.org/ 2 April 2008
Kaiser WJ (1997) Inter- and intranational spread of ascochyta pathogens of chickpeas, faba bean, and lentil. Can J Plant Pathol 19:215–224
Larena I, Salazar O, González V, Julián MC, Rubio V (1999) Design of a primer for ribosomal DNA internal transcribed spacer with enhanced specificity for ascomycetes. J Biotechnol 75:187–194
Morin L, Evans KJ, Sheppard AW (2006) Selection of pathogen agents in weed biological control: critical issues and peculiarities in relation to arthropod agents. Aust J Entomol 45:348–364
Morley TB, Morin L (2008) Progress on boneseed (Chrysanthemoides monilifera subsp. monilifera (L.) Norlindh) biological control: the boneseed leaf buckle mite Aceria (Keifer) sp., the lacy-winged seed fly Mesoclanis magnipalpis Bezzi and the boneseed rust Endophyllum osteospermi (Doidge) A.R.Wood. Plant Prot Q 23:29–31
Pedersen EA, Morrall RAA, McCartney HA, Fitt BDL (1994) Dispersal of conidia of Ascochyta fabae f.sp. lentis from infected lentil plants by simulated wind and rain. Plant Pathol 43:50–55
Perfect SE, Green JR (2001) Infection structures of biotrophic and hemibiotrophic fungal plant pathogens. Mol Plant Pathol 2:101–108
Shenoy BD, Jeewon R, Hyde KD (2007) Impact of DNA sequence-data on the taxonomy of anamorphic fungi. Fungal Divers 26:1–54
Simmons EG (1986) Alternaria themes and variations (22–26). Mycotaxon 25:287–308
Sutton BC (1977) Coelomycetes VI. Nomenclature of generic names proposed for Coelomycetes. Mycological Papers 141
Sutton BC (1980) The coelomycetes. CMI, Kew
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Thomas J, Leys A (2002) Strategic management of bitou bush (Chrysanthemoides monilifera ssp. rotundata (L.) T. Norl.). In: Spafford Jacob H, Dodd J, Moore JH (eds) Proceedings of 13th Australian Weeds Conference. Plant Protection Society of Western Australia, Perth, pp 586–590
Wakefield EM (1922) Fungi exotici. XXVI. Bulletin of Miscellaneous Information, Kew 1922(5):161–165
Weiss PW, Adair RJ, Edwards PB, Winkler MA, Downey PO (2008) Chrysanthemoides monilifera subsp. monilifera (L.) T.Norl. and subsp. rotundata (DC.) T.Norl. Plant Prot Q 23:3–14
Wood AR (2002) Infection of Chrysanthemoides monilifera by the rust fungus Endophyllum osteospermi is associated with a reduction in vegetative growth and reproduction. Australas Plant Pathol 31:409–415
Wood AR (2006) Preliminary host specificity testing of Endophyllum osteospermi (Uredinales, Pucciniaceae), a biological control agent against Chrysanthemoides monilifera ssp. monilifera. Biocontrol Sci Tech 16:495–507
Wood AR, Crous PW (2005) Epidemic increase of Endophyllum osteospermi (Uredinales, Pucciniaceae) on Chrysanthemoides monilifera. Biocontrol Sci Tech 15:117–125
Zhang Y, Fournier J, Pointing SB, Hyde KD (2008) Are Melanomma pulvis-pyrius and Trematosphaeria pertusa congeneric? Fungal Divers 33:47–60
Acknowledgements
This project would not have been possible without the support of the respective organisation of each co-author. We are grateful to all stakeholders who kindly collected and sent diseased material. We also wish to acknowledge the NSW Department of Environment and Climate Change (DECC) for financially and logistically supporting L. Morin’s visit to the Coffs Harbour area in March 2008. Thanks are due to Mr Martin Smith (DECC) for his relentless interest and support in collecting field material and to Ms Hillary Cherry (DECC) for her efforts in encouraging stakeholders to collect diseased specimens. Thanks are also extended to Mr Tom Morley (Department of Primary Industries Victoria) for providing boneseed seeds, Ms Sue Thompson (Queensland Department of Primary Industries and Fisheries) for seeds of sunflower, Mr Alexander Roberts (Australian Quarantine Inspection Services) for seeds of W. biflora, Dr John Hosking (Department of Primary Industries NSW) for seeds of S. pinnatifolius and Dr John Scott (CSIRO Entomology) for seedlings of V. dealbatum. We are also grateful to Dr Don Gomez of CSIRO Entomology and two anonymous reviewers for their valuable comments on a draft of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morin, L., Shivas, R.G., Piper, M.C. et al. Austropleospora osteospermi gen. et sp. nov. and its host specificity and distribution on Chrysanthemoides monilifera ssp. rotundata in Australia. Fungal Diversity 40, 65–74 (2010). https://doi.org/10.1007/s13225-009-0007-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13225-009-0007-7