Abstract
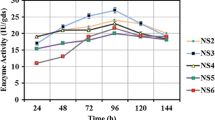
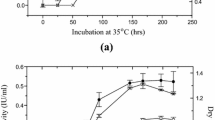
The potential of 12 fungal strains to produce carboxymethyl cellulase (CMCase) and protease on Eichhornia crassipes (water hyacinth) wastes was investigated under conditions of solid state fermentation. Ulocladium botrytis (Preuss) was selected as the best fungus for the production of both enzymes. The best nitrogen sources for production of CMCase and protease were yeast extract and malt extract, respectively. CMCase and protease were purified by isopropanol (1:1) precipitation and column chromatography on Sephadex G-100 and DEAE-cellulose. Purification fold of 47.34 and 51.78, with 852.11 and 1,469.38 U/mg specific activity was achieved with 40.3 and 56.25% recovery after purification of CMCase and protease, respectively. The purified CMCase expressed its maximal activity at 60°C and pH 5.2, showed good stability in the pH range of 5.2–5.4 and its midpoint of thermal inactivation (Tm) was 60°C after 75 min exposure. The purified protease expressed its maximal activity at 35°C and pH 5.2, showed good stability in the pH range of 5.6–6.0 and its midpoint of thermal inactivation (Tm) was 35°C after 75 min exposure. The best substrate concentration for CMCase was 1.2% (w/v) Na-CMC and for protease, it was 0.8% (w/v) casein. The best enzyme concentration for the two tested enzymes was 0.4 U/ml. Ions of Ca2+, Na+ and K+ showed a stimulatory effect but sodium arsenate and iodoacetate showed an inhibitory effect. Moreover, Ag2+ and Hg2+ inhibited both enzyme activities completely. The purified enzymes from Ulocladium botrytis had molecular weights of 50 and 83 kDa for CMCase, and 47 kDa for protease on SDS-PAGE.






Similar content being viewed by others
References
Abd El-Rahman EM (1990) Studies on some thermophlic bacterial strains. PhD Thesis, Al-Azhar University, Cairo
Abd-el-Naby MA (1988) Biochemical study on fungal cellulase. PhD thesis, Faculty of Science, Mansoura University
Abdul-Raouf UM (1990) Studies of proteolytic bacteria isolated from certain localities in Aswan city. MSc thesis, Al-Azhar University, Cairo
Alagarsamy S, Chandran S, George S, Carlos RS, Ashok P (2005) Production and partial purification of a neutral metalloproteaseby fungal mixed substrate fermentation. Food Technol Biotechnol 43:313–319
Ali UF, Saad El-Dein H (2008) Production and partial purification of cellulose complex by Aspergillus niger and A. nidulans grown on water hyacinth blend. J Appl Sci Res 4(7):875–891
Ammar MS, Bayoumi RA, El-Kasaby AMH, Soliman AM (2003) Purification and properties of thermostable protease by B. brevis geltinoamylolyticus using fish wastes (Fi W) and poultry wastes (Po W) under solid state fermentation conditions. 5th Int Sic Conf Al-Azhar Univ Fac Sci 25–27, Egypt, p 54
Bayoumi RA, Yassin HM, Swelim MA, Abdel-All EZ (2008) Production of bacterial pectinase(s) from agro-industrial wastes under solid state fermentation conditions. J Appl Sci Res 4(12):1708–1721
Bhat MK (2000) Cellulases and related enzymes in biotechnology. Biotechnol Adv 18:355–383
Calvert P (2002) Water hyacinth control and possible uses. Technical Brief International Technology Development Centre UK. Cellulose ethanol is ready to go (2004) News release, April 21, Iogen Corporation, Canada
Coral G, Arikan B, Unaldi MN, Guvenmes H (2002) Some properties of crude carboxymethyl cellulase of Aspergillus niger Z10 wild type strain. Turk J Biol 26:209–213
Datta A (1992) Purification and characterization of a novel protease from solid substrate cultures of Phanerochaete chrysosporium. J Biol Chem 267:728–736
Deshpandel P, Nair S, Khedkar S (2008) Water hyacinth as carbon source for the production of cellulase by Trichoderma reesei. Appl Biochem Biotechnol 10:8476–8489
Dixon M, Webb EC (1979) Enzyme kinetics. In: Enzymes, 3rd edn. Academic, New York, p 47
El-Gindy AA, Ali UF, Ibrahim ZM, Isaac GS (2008) A costeffective medium for enhanced production of extracellular α-galactosidase in solid substrate cultures of Aspergillus awamori and A. carbonarius. Aust J Basic Appl Sci 2(4):880–899
El-Safey EM (1994) Production of microbial α-amylases under solid-state incubation conditions in the open air. MSc thesis, Al-Azhar University, Cairo
El-Safey EM, Ammar MS (2003) Purification and characterization of NH- α-amylase isolated from Aspergillus flavus var. columnaris. International Confernces of Enzymes in The Environment, Activity, Ecology And Applications, Praha, Czech Republic, 14–17 July, pp 127
Evans DA, Bravo JE (1983) Plant protoplast isolation and culture. Int Rev Cytol Suppl 16:33–53
Garg AP, Sudha G, Mukerji KG, Pugh GJF (1985) Ecology of keretenophilic fungi. Proc Ind Acad Sci Plant Sci 94:194–163
Glantz AS (1992) Primer of biostatistics. McGraw Hill, New York, pp 2–18
Gopal B (1987) Water hyacinth. Aquatic plant studies series. Hindasia, New Delhi
Gunnarsson CC, Petersen CM (2007) Water hyacinths as a resource in agriculture and energy production: a literature review. Waste Manage 27(1):117–129
Johnvesly B, Naik GR (2001) Studies on the production of thermostable alkaline protease from thermophilic and alkaliphilic Bacillus sp. JB-99 in a chemically defined medium. Process Biochem 37:139–144
Joo HS, Kumar CG, Park GC, Kim KT, Paik SR, Chang CS (2002) Optimization of the production of an extracellular alkaline protease from Bacillus Horikoshii. Process Biochem 38:155–159
Kim BK, Lee BH, Yoo JL, Hyuck J, Chung HC, Jin WL (2009) Purification and characterization of carboxymethyl cellulase isolated from a marine bacterium, Bacillus subtilis subsp subtilis A-53. Enzyme Microb Technol 44(67):411–416
Kunitz M (1947) Crystalline soybean trypsin inhibitor, II General properties. J Gen Tokyo 46(10):291–310
Laemmli UK (1970) Cleavage of structural protein during the assembly of head of bacteriophage T4. Nature 227:680–685
Lee CY, Cheng MF, Yu MS, Pan MJ (2002) Purification and characterization of a putative virulence factor, serine protease, from Vibrio parahaemolyticus. FEMS Microbiol Lett 209(1):31–37
Louboudy SS, El-Gamal MS, Ammar MS, Ali MO (2001) Microbial utilization of Eichhornia crassipes for pectinases and cellulases enzyme production under solid state fermentation (SSF) conditions. Fourth Int Sci Conf “Science Development and Environment”. Science Fac, Al-Azhar Univ, Cairo, Egypt, 27–29 March p 32
Lowery OH, Resenbrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mahmoud SAZ, Taha SM, Attia RM (1968) Effect of metal ion activators on the reaction velocity of bacterial alpha amylase. J Bot UAR 11:41–48
Mehdi D, Heidi S, Wensheng Q (2009) Fungal bioconversion of lignocellulosic residues; opportunities and perspectives. Int J Biol Sci 5(6):578–595
Miller GL (1959) Use of dinitrosalicyclic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Moor-Landecker E (1990) Fundamentals of the fungi. Prentice Hall, Englewood Cliffs, NJ
Nagendra PG (2001) Strategies for economic utilisation of aquatic weeds of Kerala. Proceedings of the National Seminar on Kuttanad Development Edathua, Alleppey, India
Nehra KS, Singh A, Sharma J, Kumar R, Dhillon S (2004) Production and characterization of alkaline protease from Aspergillus species and its compatability with commercial detergents. Asian J Microbiol Biotechnol Environ Sci 6:67–72
Nongporn HT, Anongnat P, Prasert S (1999) Purification and characterization of an extracellular protease from alkaliphilic and thermophilic Bacillus sp. J Biosci Bioeng 87(5):581–587
Palmer T (1991) Extraction and purification of enzymes. In: Understanding Enzymes. Ellis Horwood, Ltd, England, pp 301–317
Patel R, Dodia M, Singh SP (2005) Extracellular alkaline protease from a newly isolated haloalkaliphilic Bacillus sp: production and optimization. Process Biochem 40:3569–3575
Peterson EA, Sober HA (1962) Column chromatography of protein: substituted cellulases. In: Colowich S, Kaplan N (eds) Methods in enzymology, vol 5. Wiley, New York, pp 3–27
Plummer DT (1978) The practice of column chromatography. In: An introduction to practical biochemistry. McGraw-Hill, New York, pp 61–66
Po-Jui C, Tao-Chun W, Yao-Tsung C, Liang-Ping L (2004) Purification and characterization of Na-CMC from Sinorhizobium fredii. Bot Bull Acad Sin 45:111–118
Potrykus I, Shillito RD (1986) Protoplasts: isolation, culture, plant regeneration. Methods Enzymol 118:549–578
Roy U, Vora VC (1989) Purification and properties of a carboxymethyl cellulase from phytopathogenic fungus macrophomina phaseolina. Indian J Biochem Biophys 26(4):243–248
Roy SK, Dey SK, Raha SK, Chakrabatry SL (1990) Purification and properties of an extracellular endoglucanase from Myceliophthora thermophila. J Gen Microbiol 136:1967–1971
Tsuchiya K, Arai T, Seki K, Kimura T (1987) Purification and some properties of alkaline protease from Cephalosporium sp. KM 338. Agric Biol Chem 51:2959–2965
Usama FA, Saad El-Dein HS (2008) Production and partial purification of cellulase complex by Aspergillus niger and A. nidulans grown on water hyacinth blend. J Appl Sci Res 4: 875–891
West ES, Tood WR, Mason HS, Van Burggen JT (1967) Text book of biochemistry, 4th edn. MacMillan, London
Yang J, Shih I, Tzeng Y, Wang S (2000) Production and purification of protease from a Bacillus subtilis that can deproteinize wastes. Enzyme Microb Technol 26:406–413
Acknowledgment
The authors wish to express their deepest gratitude to Prof. Dr. Ahmed Fouad Afifi, Professor of Microbiology and Formerly Head of Biological Sciences Department, Faculty of Education, Ain Shams University and Dr. Eman M. Fawzy associate professor of microbiology for their useful criticism and continuous encouragement.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abo-Elmagd, H.I., Housseiny, M.M. Purification and characterization of carboxymethyl cellulase and protease by Ulocladium botrytis Preuss ATCC 18042 using water hyacinth as a substrate under solid state fermentation. Ann Microbiol 62, 1547–1556 (2012). https://doi.org/10.1007/s13213-011-0409-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-011-0409-0