Abstract
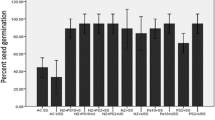
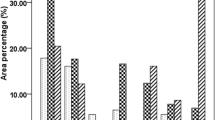
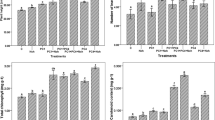
The present study evaluated the effect of nanochitosan in combination with plant growth promoting rhizobacteria (PGPR), PS2 and PS10 on maize growth. The PGPR were earlier recognized as Bacillus spp. on the basis of 16S rDNA sequencing. The observation revealed enhanced plant health parameters like seed germination (from 60 to 96.97%), plant height (1.5-fold increase), and leaf area (twofold). Variability in different physicochemical parameters (pH, oxidizable organic carbon, available phosphorous, available potassium, ammoniacal nitrogen and nitrate nitrogen) was observed. Activities of soil health indicator enzymes (dehydrogenase, fluorescein diacetate hydrolysis and alkaline phosphatase) were also enhanced 2 to 3 fold. Plant metabolites with respect to different treatments were also analyzed using gas chromatography–mass spectroscopy (GC–MS) and the result revealed an increase in the amounts of alcohols, acid ester and aldehyde compounds. Increase in organic acids indicates increased stress tolerance mechanism operating in maize plant after treatment of nanochitosan.






Similar content being viewed by others
Abbreviations
- NC:
-
Nanochitosan
- SS:
-
Sterilized soil
- USS:
-
Unsterilized soil
References
Aminiyan MM, Sinegani AAS, Sheklabadi M (2015) Aggregation stability and organic carbon fraction in a soil amended with some plant residues, nanozeolite, and natural zeolite. Int J Recycl Org Waste Agric 4(1):11–22
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenol oxidase in Beta vulgaris. Plant Physiol 24:1–15
Casida L, Klein D, Santoro T (1964) Soil dehydrogenase activity. Soil Sci 98:371–376
Glenn AR (1976) Production of extracellular proteins by bacteria. Annu Rev Microbiol 30:41–62
Gonzalez P, Neilson RP, Lenihan JM, Drapek RJ (2010) Global patterns in the vulnerability of ecosystems to vegetation shifts due to climate change. Glob Ecol Biogeogr 10:1–14
Goto M, Ehara H, Karita S, Takabe K, Ogawa N, Yamada Y, Ogawa S, Yahaya MS, Morita O (2003) Protective effect of silicon on phenolic biosynthesis and ultraviolet spectral stress in rice crop. Plant Sci 164:349–356
Kandeler E (1996) Nitrate. In: Schinner F, Öhlinger R, Kandeler E, Margesin R (eds) Methods in soil biology. Springer, Berlin, pp 408–410
Kloepper JW, Schroth MN, Miller TD (1980) Effect of rhizosphere colonization by Plant Growth promoting rhizobacteria on Potato Plant development and Yield. Phytopathology 70:1078–1082
Li B, Liu B, Su T, Fang Y, Xie G, Wang G, Wang Y, Sun G (2010) Effect of chitosan solution on the inhibition of Pseudomonas fluorescens causing bacterial head root of broccoli. Plant Pathol J 26:189–193
Lu CM, Zhang CY, Wen JQ, Wu GR, Tao MX (2002) Research of the effect of nanometer materials on germination and growth enhancement of Glycine max and its mechanism. Soybean Sci 21:168–172
Maynard AD, Aitken RJ, Butz T, Colvin V, Donaldson K, Oberdörster G, Philbert MA, Ryan J, Seaton A, Stone V, Tinkle SS, Walker L, Warheit NJ, Warheit DB (2006) Safe handling of nanotechnology. Nature 444:267–269
Meng X, Yang L, Kennedy JF, Tian S (2011) Effects of chitosan and oligochitosan on growth of two fungal pathogens and physiological properties in pear fruit. Carbohyd Polym 81:70–75
Mukhopadhyay SS (2014) Nanotechnology in agriculture: prospects and constraints. Nanotechnol Sci Appl 7:63–71
Namasivayam SKR, Chitrakala K (2011) Ecotoxicological effect of Lecanicillium lecanii (Ascomycota: Hypocreales) based silver nanoparticles on growth parameters of economically important plants. J Biopestic 4(1):97–101
Nel A, Xia T, Mädler L, Li N (2006) Toxic potential of materials at the nano level. Science 311(5761):622–627
Purvis AC (1980) Sequence of chloroplast degreening in calamondin fruit as influenced by ethylene and AgNO3. Plant Physiol 66:624–627
Schnurer J, Rosswall T (1982) Fluorescein diacetate hydrolysis as a measure of total microbial activity in soil and litter. Appl Environ Microbiol 6:1256–1261
Seif SM, Sorooshzadeh AH, Rezazadeh S, Naghdibadi HA (2011) Effect of nano silver and silver nitrate on seed yield of borage. J Med Plant Res 5(2):171–175
Shukla SK, Mishra AK, Arotiba OA, Mamba BB (2013) Chitosan-based nanomaterials: a state of the art review. Int J Biol Macromol. doi:10.1016/j.ijbiomac.2013.04.043
Stepanova AN, Yun J, Likhacheva AV, Alonso JM (2007) Multilevel interactions between ethylene and auxin in Arabidopsis roots. Plant Cell 19:2169–2185
Tabatabai MA, Bremner JM (1969) Use of p-nitrophenylphosphate for assay of soil phosphatase activity. Soil Biol Biochem 1:301–307
Urbonaviciute A, Samuoliene G, Sakalauskaite J, Duchovskis P, Brazaityte A, Siksnianiene JB, Ulinskaite R, Sabajeviene G, Baranauskis K (2006) The effect of elevated CO2 concentrations on leaf carbohydrate, chlorophyll contents and photosynthesis in radish. Pol J Environ Stud 15:921–925
Vinhal-Freitas IC, Wangen DRB, Ferreira AS, Corrêa GF, Wendling B (2010) Microbial and enzymatic activity in soil after organic composting. Revista Brasileira de Ciência do Solo 34:757–764
Yoshida S, Forno DA, Cock JH (1972) Laboratory Manual for Physiological studies of rice. International Rice Research Institute. 2nd Ed
Zheng L, Hong F, Lu S, Liu C (2005) Effect of nano-TiO2 on strength of naturally aged seeds and growth of spinach. Biol Trace Elem Res 104:83–91
Zhou DM, Jin SY, Wang YJ, Wang P, Weng NY, Wang Y (2012) Assessing the impact of iron-based nanoparticles on pH. Dissolved Org Carbon Nutr Availab Soils Soil Sediment Contam Int J 21(1):101–114
Acknowledgements
The author acknowledges UGC India for the funding support in form of JRF and SRF and Dept. of Microbiology for providing research facilities.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest for this manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Khati, P., Chaudhary, P., Gangola, S. et al. Nanochitosan supports growth of Zea mays and also maintains soil health following growth. 3 Biotech 7, 81 (2017). https://doi.org/10.1007/s13205-017-0668-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-017-0668-y