Abstract
Environmentally friendly municipal solid waste management is the biggest problem facing several developing countries, including Ghana. Heavy metals pollution generated by landfill leachate has become increasingly concerned due to its potential impact on human health. This study assessed the pollution level and sources of heavy metal levels in groundwater, as well as evaluated the human health risk effect. The sampling technique and sample treatment were done based on the Standard Methods for the Examination of Water and Wastewater. The results suggested that the mean concentration of Pb, Fe, Cd, and Cr was above the acceptable limits of the World Health Organization for drinking water except for Zn and Cu. The heavy pollution index indicates contamination, while hazard index values at sites BH1 and W4 were greater than one, suggesting adverse health effects. However, the heavy metal pollution index values were less than the critical limit of 100 for drinking water. Multivariate analysis predicted that lithogenic and anthropogenic factors were the possible sources of water pollution of heavy metal in the Oti community. Thus, multivariate statistical techniques could be a beneficial tool for the evaluation of possible sources of heavy metal contamination. The high levels of heavy metals found in the Oti community suggested a considerable pollution of water by leachate percolation from the landfill site. The findings of the study, which can be used in areas under similar environmental conditions, can offer a valuable benchmark for the design of suitable approaches to manage groundwater resources by both local and national policymakers.
Similar content being viewed by others
Introduction
Man’s activities from manufacturing and processing of consumption lead to waste generation. Management of municipal solid waste (MSW) is one of the major environmental and public health problems for major cities in the developing countries (Renou et al. 2008). Among the waste management methods, landfill is the inexpensive, simple, and most economical way of managing solid wastes. In particular, developing countries use landfills as the primary method for disposing of innocuous solid waste because of their considerable advantages, such as low technological barriers and economic efficiency (Gonzalez-Valencia et al. 2016). However, several landfills in developing countries are operated below acceptable standards (Oyeku and Eludoyin 2010) and the wastes are not sorted; hence, leachates and toxic gases are accidentally released into the environment (Alimba et al. 2012; Schrapp and Al-Mutairi 2010). Thus, in developing countries, it is common to find unhygienic landfills in public places (i.e., close to residential buildings) and seasonal high-water table areas (Alimba et al. 2006).
Leachates are generated when MSW encounters water that penetrate through the landfill sites. The exposure to the leachate constituents above the recommended limits can be associated with a plethora of biometal poisoning-related symptoms and diseases, for example, asthma, depression, vomiting and convulsion, ataxia, cardiovascular and renal diseases, diarrhea, neurological diseases, cancer, hypertension, pneumonitis, skeletal deformities, anemia, and gastrointestinal disorders (Abarikwu et al. 2013; Farombi et al. 2012). Leachate can leak via geomembranes and enter into the soil and aquatic environments through manufacturing and construction defects, as well as a vapor diffusion via the liner (Grugnaletti et al. 2016; Pantini et al. 2014). Therefore, areas close to landfills have a greater chance of water pollution due to the possible contamination source of leachate originating from the dump sites. Thus, leakage of leachate can cause pollution of nearby groundwater and surface water, agriculture, and natural ecosystems, especially when the leachate is released uncontrolled, and hence, can cause environmental health issues in many developing countries (Adamcová et al. 2017; Oyeku and Eludoyin 2010; Samadder et al. 2017). The effect of landfill leachate in surface water and groundwater has been reported (Abu Qdais 2010; Guan et al. 2014). Conventionally, groundwater is regarded as a good natural quality due to its geological environment (MacDonald and Calow 2009).
Landfill leachate is a concern since it is a complex mixture composed of several pollutants, such as heavy metals, soluble inorganic and organic compounds, suspended particles, and nutrients (Mavakala et al. 2016; Naveen et al. 2017). Monitoring of heavy metal concentrations in landfill leachate has been routinely performed by landfill operators (Baun and Christensen 2004). Heavy metals present in leachates from both hazardous waste dump sites and municipal solid waste landfills pose a serious threat to public health, since they can cause several physiological effects to human health (Jaishankar et al. 2014), as well as ecotoxicological impacts on terrestrial and aquatic ecosystems (Nagajyoti et al. 2010). For example, long-term exposure to arsenic can cause neurological problems, internal cancers, cardiovascular, and hypertension disease (Smith et al. 2000). Heavy metals can also inhibit synthesis and growth of photosynthetic pigments on the Lemna gibba (Demim et al. 2013). This issue also even deteriorated with the improper disposal of electronic products, leading to the release of high levels of heavy metals (Awasthi et al. 2016; Wittsiepe et al. 2017).
The quantity and quality of leachate are influenced by several factors, such as the composition of the waste, biochemical processes that occur in the degradation stages of the waste, amount of moisture, and the local parameters (Ma et al. 2018). The composition of leachate formed varied significantly based on the biological and chemical reactions on solid waste, the age of the landfill, waste compositions, landfilling technology, and climatic conditions (Kjeldsen et al. 2002; Ziyang et al. 2009). Various treatment approaches have been used for removing organic contaminants or heavy metals from landfill leachate and wastewater. Effective removal of heavy metals from wastewater was achieved through micellar-enhanced ultrafiltration (Landaburu-Aguirre et al. 2009) and biosorption (Souiri et al. 2009). Modern high-rejection reverse osmosis membranes can also retain both dissolved inorganic and organic contaminants with rejection rates as high as 98–99% (Peters 1998). Efficient removal of heavy metals through adsorption onto composite materials (Mojiri et al. 2016), activated carbon (Foul et al. 2009), and chitosan/montmorillonite (Assaad et al. 2007) has also been reported. Advanced oxidation processes have also been employed for removing organic contaminants in wastewater (Chemlal et al. 2014).
Solid waste management has become a tremendous work for the densely populated developing country due to the huge production rate of wastes and poor management of infrastructure. The issue of MSW is more serious in developing countries, including Ghana, where MSW disposal is mostly operated using dump yards or landfills that lack leachate collection and treatment systems. In Ghana, the production of MSW has developed in parallel with rapid population growth, as well as urban, commercial, and industrial expansion. Moreover, the management systems of industrial and urban waste are limited, and landfills considered to be disposal sites are situated in urban rivers, agricultural lands, and public spaces without treatment. Considering the different types of soil contamination, heavy metals are considered one of the most challenging environmental issues because of their persistence, non-biodegradability, toxicity, and bioaccumulation (Alloway 2013). Heavy metal pollution of groundwater and surface water surrounding the landfill sites has identified as the most severe environmental issues in many developing countries including Ghana (Kumar and Alappat 2005; Longe and Balogun 2010; Nartey et al. 2012; Nyame et al. 2012; Osei et al. 2011). The majority of earlier studies focus on assessing heavy metals pollution hazards in groundwater environments due to their toxicity (Azizi et al. 2015; Cheng et al. 2013; Huang et al. 2011; Kucuksezgin et al. 2006). For instance, Cheng et al. (2013) determine the health hazards of heavy metals, such as Mn, Cr, Pb, Cu, Fe, Ni, and Zn, in the aquaculture pond ecosystem of Pearl River Delta, China, via the health risk assessment approach. Huang et al. (2011) adopt the potential ecological risk index and geo-accumulation index approaches to assess the extent of pollution from heavy metals, such as Ni, Cr, Cd, Zn, and Pb, present in liquefaction residues of sewage sludge.
In Ghana, the composition of solid waste varies significantly as all types of waste, such as redundant vegetables, broken batteries, food, thermometers, plastics bags, and chemical waste, are discarded together without proper separation. Since 2004, most of the MSW in Kumasi Metropolis has been directly deposited at the Oti landfill, Dompoase, without any bottom sealing systems in place. The Oti landfill at Dompoase was sited far from residential areas in 2004 when it was opened. However, rapid population growth and urbanization have caused the number of residential buildings and other infrastructures to increase exponentially. Thus, residential buildings are now encroaching proximally to the Oti landfill site. About 1000 and 1200 metric tons of waste from Kumasi is dumped daily. This untreated fecal matter ends up in other communities such as Esreso, Adagya, Sokoban, Odaho, Ntenanko, Barekese, Bekwai, and Anwia Nkwanta where the Oda River flow through. To prevent soil, groundwater, and surface water contamination, the use of an engineered barrier system in modern landfill sites is a growing concern in most developing countries. Landfilling technology has undergone many advancements in the last decades and evolved from uncontrolled city dumps to highly engineered structures designed to protect the environment. Despite these improvements, it has been reported that even sanitary landfill sites, which are commonly used to dispose of waste in a scientific manner in urban areas, have the potential to pollute the surface and groundwater.
Up to now, the provision of portable water is a major issue, particularly in many developing countries, including Ghana (Ahaneku and Adeoye 2014). The demand for good drinking water in Ghana has increased in the past decades without any rise in quality and quantity. Because of the above issues, several households in the Oti community have resulted in using boreholes and hand-dug wells as the only source of water for drinking and domestic purposes, where many people who reside near landfill sites are not aware of the health effects of drinking leachate-contaminated groundwater. One of the major environmental challenges of several developing countries is the absence of a well-coordinated management policy to check the inappropriate disposal of solid waste into the environment. It is common to see heaps of solid waste deface the landscape of most cities in Ghana and coupled with the fact that most Ghanaians use open disposal devices that could ultimately pose serious hazards on human health. To prevent the hazard posed by the landfill leachates, precautionary facilities must be constructed to cater for both active and inactive leachates. The aim of this study was to assess the health risk related to heavy metal pollution of groundwater for drinking in the Oti community. This study makes the use of pollution evaluation indices and multivariate statistical technique as a complementary tool to check the likely source of contamination, which affects the quality of water in the study area. This study will help policymakers in combating contamination around landfill sites by identifying the major pollution source.
Materials and methods
Study area
The Kumasi Metropolis lies within latitude 6.35°–6.40′N and longitude 1.30°–1.35′W and occupies an area of 254 km2. The middle Precambrian Rock is the main geological formation in the Kumasi Metropolis. The major soil type in the area is mainly the forest Ochrosol. The soils in some peri-urban areas are developed on phyllites or granites. Those develop on phyllites are less acidic, while those of granites are acidic. The type of soil in the Kumasi Metropolis includes Gleyic Cambisols, Gleyic Arenosols, Eutric Gleysols and Haplic Acrisols with Ferric Acrisols as the most common soil type (Mohammed 2004). The aquifer is a sandy shallow type of about 7–10 m thickness. Soil recovered from the borehole drillings and hand-dug well reveals that the soil strata at the top of the landfill were covered with rubbish. The climate is a wet sub-equatorial type with a double maximum rainfall regime of about 214.3 mm in June and 165.2 mm in September every year. The average temperature ranges between 21.5 and 30.7 °C, with the average annual humidity of about 86%. The metropolis is in the moist semi-deciduous southeast ecological area. The rich soil in the Kumasi Metropolis has promoted agriculture. The city has a special place in terms of the economic, social, political, and cultural life of Ghana. Kumasi Metropolis has about 2.5 million populations owing to travelers from neighboring districts that come to conduct business activities. Roughly half of the waste produced in Kumasi originates from households, while the rest is contributed by industrial institutional and commercial sources. The city supports a wide range of products, which include plastics, textiles, telecommunication components, electronics, and biomedical products. As a result of the manufacturing activities in the city and a large residential population, heavy metals are likely to present in the landfill leachate. The Oti landfill is one of the sanitary landfills in Kumasi Metropolis. The Oti landfill site was owned by the Kumasi Metropolitan Assembly and operated by J. Stanley-Owusu and Company Limited. The Oti landfill site serves as a solid waste disposal site with a septage treatment plant attached. The Landfill commenced operation in 2004, which was designed for a period of 15 years. This was developed in three phases for every 5 years. The Oti landfill has a size of about 1000 acres and serves as the only landfill for the Kumasi Metropolis made up of nine sub-metros and one (1) municipal generate about 1500 metric tons of waste daily, out of which 1400 is collected with the rest finding their way into drains, gutters, and open places. Constructed as a waste stabilization pond, which takes care of liquid waste, the site has been managed run by the J. Stanley-Owusu and Company Limited, but the company had for some time now abandoned the site, because it is owed millions of cedis in management fees by the Kumasi Metropolitan Assembly. People have built close to the landfill despite the environmental and health threats that come with living close to such sites. This landfill site has been used as an uncontrolled open system without any engineering operations on the flat area and/or the surrounding farmland. The site received municipal solids and liquid wastes generated from commercial activities, hospitals, urban municipal, and industry residues. The leachate generated from the uncontrolled open system without any engineering operations flows into the cropland and groundwater tables around the site. The lack of a barrier system or a leachate collection system in the Oti landfill results in the release and flow of leachate into the surrounding groundwater resources and cropland. In September 2016, some residents protest on the improper management of the Oti landfill site, which the demonstrators claimed a released of pungent smell all over the community.
Chemicals and reagents
All chemicals utilized were of high analytical reagent grade, which was supplied by BDH Chemical Ltd., UK, and Sigma-Aldrich Chemie GmbH, Steinheim, Germany. Sampling containers and glassware were rinsed with 1% HCl, saturated in 10% HNO3, and then rinsed with deionized water before use.
Sampling and preparation
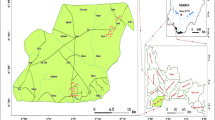
Triplicate monthly samples of treating and untreated leachate around the dumpsite were collected from three different locations (L1, L2, and L3). The Oti landfill site is subdivided into three sections: L1, L2, and L3. The L1, L2, and L3 were chosen as the point before the treatment process, the point of entry of treated effluent, and point after the treatment process, respectively. To comprehend the temporal and spatial variations in the geochemistry of landfill leachates, the leachate sample points were selected based on the natural streams draining the area, flow regimes, areas close to the dumpsite, and topography. The leachates samples were sampled at three sampling points of the treatment plant. The locations L1 and L3 points were located at the start and end, respectively, of the drainage channel of the treatment plant, while the L2 point was in the middle (Fig. 1).
The leachate-affected water sampling points are selected based on the groundwater movement of the region, which mainly occurs southwest to the northeast. Three boreholes and four hand-dug wells were selected from the Oti community to collect groundwater samples, as shown in Fig. 1. The boreholes and hand-dug well were labeled as BH1, BH2, and BH3 and the W1, W2, W3, and W4, respectively. The boreholes and hand-dug well were well situated and located on the pathway of the groundwater flow in such a way that any contaminants in the groundwater will be captured. Moreover, the ability to sample all planned water samples was affected by poor access and dynamic functional status to some of the boreholes and hand-dug well samples of the study area. A global positioning system (Garmin 76) was used to record each sampling location (Table 1).
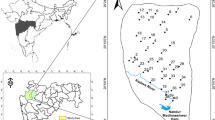
Forty-two water samples were fetched from hand-dug wells and boreholes between December 2016 and March 2017. For boreholes, the water samples were fetched after pumping for 15 min to eliminate immobile water. The samples were collected into a clean 1-L capacity polyethylene bottles and acidified with HNO3 to a pH < 2.0 to minimize adsorption and precipitation on the container wall (APHA 2005). The groundwater was found to enter the study area from the east–south and flow toward the northwest direction. The leachate was assumed to follow the groundwater flow direction. Figure 2 shows the groundwater direction to the water table data.
Heavy metal analysis
Heavy metal concentrations (Cu, Cr, Cd, Pb, Zn, and Fe) in the groundwater samples were measured using Buck Scientific model 210 VGP Atomic Absorption Spectrophotometer with deuterium background correction lamp. A standard curve was obtained by running a prepared standard solution of each heavy metal. Hundred milliliters of each sample was transferred into a beaker. A 5-mL conc. HCl was added and heated using a hot plate until the volume was reduced to 20 mL. The sample was cooled and then filtered. The pH of the digest sample was adjusted to 4 by adding 5.0 N NaOH. The sample was transferred to a 100-mL volumetric flask and then diluted to the mark with deionized water. The digest samples were used for the heavy metal analysis. A standard solution containing a 1000 mg/L of 2% HNO3 was used to prepare the spiking experiments and calibration standards. Three working standards of each heavy metal were prepared from these standards.
Quality control
The quality of measured values and analytical methods were verified by analyzing a certified reference material (SRM 1640a), standards, and blank sample. Replicate analysis of this reference material exhibited good accuracy with a relative standard deviation of ≤ 4% and recovery rate between 94.5 and 105.8%.
Estimation of pollution evaluation indices in the water samples
To comprehend the overall water quality with reference to the selected heavy metals, the heavy metal pollution index (HPI), heavy metal evaluation index (HEI), and degree of contamination (Cd) were used.
Heavy metal pollution index
The HPI represents the total water quality relative to the heavy metal. The HPI was inversely proportional to the standard of the resultant heavy metal (Horton 1965). The relative weight (Wi) was inversely proportional to the World Health Organization (WHO) standard (WHO 2011). The HPI was evaluated following Eq. (1) (Mohan et al. 1996):
where Wi is the unit weight of the ith parameter, Qi is sub-index of the ith parameter, and n is the number of parameters considered in this study. The Qi is determined following Eq. (2) (Mohan et al. 1996):
where Ii and Mi are the ideal values and heavy metals concentration of the ith parameter, respectively (Mohan et al. 1996).
Heavy metal evaluation index
According to Edet and Offiong (2002), HEI offers information on the overall water quality relative to heavy metal, which was calculated according to Eq. (3):
where Hmac and Hc are the maximum admissible concentration and monitored value of the ith parameter, respectively (Edet and Offiong 2002).
Degree of contamination
According to Backman et al. (1998), the degree of contamination was a summation of the combined effect of the water quality parameters, which was regarded toxic to consumers and was determined following Eq. (4):
where N represents the normative value and CNi is the maximum admissible concentration. CNi and CAi represent the upper permissible concentration and analytical value of the ith component, respectively.
Health risk assessment
Risk assessment parameters and methods by Wongsasuluk et al. (2014) were utilized in this study. According to Lee et al. (2005), health risk assessment comprises dose–response, exposure assessment, hazard identification, and risk characterization. Siriwong (2006) calculated the average daily dose (ADD) of each heavy metal following Eq. (5):
All the parameters used in Eq. (5) are defined in Table 2 with the values valid on the assumption that the people in Oti community drink from the borehole and well water samples.
The human health risk of metals in the water samples was assessed as non-carcinogenic using the ADD calculation as follows:
The reference dose (RfD) values for each heavy metal are presented in Table 3.
An HI < 1 signifies an acceptable level of risk, while HI > 1 represents an unacceptable risk of non-carcinogenic effects (Lim et al. 2008).
The carcinogenic risk is the possibility of an individual to develop any type of cancer during the lifetime exposure to carcinogenic threats (Li et al. 2014). According to USEPA (1989), the slope factor (SF) directly transforms the ADD of pollutants exposed over a lifetime to the continual risk of a cancer patient:
The values for SF in mg/kg-day, RfD, and other calculated parameters are presented in Table 3.
Risk value < 10−6 represents no carcinogenic risk to health, while a risk value > 1 × 10−4 suggests a high risk of developing cancer. A risk value ranging from 1 × 10−6 to 1 × 10−4 signifies an acceptable risk to human health (Hu et al. 2012).
Pollution evaluation index
To assess the heavy metals contamination of the groundwater, a single-factor pollution index proposed by Zhaoyong et al. (2015) was used:
where Ci and Si are the measured concentration and the evaluation standard of heavy metals, respectively.
Metal index
The metal index (MI) was used in this study to aid in the estimation of the overall water quality for drinking purpose. MI was evaluated following the formula proposed by Tamasi and Cini (2004), as shown in Eq. (10):
MI values greater than 1 represent a threshold warning (Lyulko et al. 2001). Table 4 presents the metal index classification proposed by Lyulko et al. (2001) of water for drinking and domestic purposes.
Statistical analysis
IBM Statistical Package for the Social Sciences (SPSS) 20.0 was used for the statistical analysis. Basic statistical parameters including range, mean, and standard deviation were analyzed alongside the correlation analysis and multivariate analysis, such as the principal components analysis (PCA). Multivariate analysis is an important method for investigating the relationship between variables and samples and can simultaneously process water quality data. PCA was used to distinguish the principal components for converting multiple parameters to a few comprehensive indexes and could replace the original parameters. Since the PCA results could be affected by the non-independence of the spectral data, the partial correlation and dependence was tested by Kaiser–Meyer–Olkin (KMO) and Bartlett’s test (Guo et al. 2014). Correlation analysis was used to study the relationship between variables. Significance levels are described as nonsignificant (p > 0.05), significant (0.05 < p < 0.01), or highly significant (p < 0.01).
Results and discussion
Heavy metals analysis of the leachate samples
Heavy metals concentrations in landfill leachate have been an important parameter for choosing a suitable leachate treatment method (Tolaymat et al. 2004). Heavy metals in leachate can take different forms depending on complexation of a waste matrix, degradation phase, and pH (Ashworth 2005). The presence of large concentrations of heavy metal can retard the stability of the solid waste degradation process (Pohland and Harper 1987). The levels of heavy metal from the leachate samples are presented in Table 5.
Levels (mg/L) for Fe, Zn, Pb, Cd, Cr, and Cu ranged from 10.885 ± 0.069 to 25.612 ± 2.855, 1.722 ± 1.324 to 6.092 ± 3.885, 1.516 ± 1.002 to 3.574 ± 2.228, 0.492 ± 0.157 to 1.083 ± 0.065, 0.701 ± 0.498 to 1.918 ± 1.900, and 1.731 ± 1.356 to 1.984 ± 0.663 mg/L, respectively. The mean concentration of Fe, Zn, Pb, Cd, Cr, and Cu was above the Ghana EPA limit of 0.03, 3, 0.01, 0.003, 1.00, and 0.05 mg/L, respectively, for wastewater (EPA-Ghana 2001). The high level of Fe at site L1 suggests that Fe and steel scrap were dumped at the landfill site. The levels of Fe from this study were higher than that of the 2.05–18.0 mg/L reported by Nyame et al. (2012) at Accra Metropolis, Ghana. However, the levels of Fe were lower than the 63.41 and 400 mg/L recorded by Nagarajan et al. (2012) and Kanmani and Gandhimathi (2013) in Tamil Nadu and Tiruchirappalli landfill, India, respectively. The presence of Zn in the leachate suggests that the landfill receives waste from fluorescent lamps and batteries (Kanmani and Gandhimathi 2013). Comparatively, the Tiruchirappalli landfills recorded higher zinc levels of 4.80 mg/L (Kanmani and Gandhimathi 2013). This high level at site L1 compared to L3 suggests the disposal of Pb pipes, batteries, Pb-based paints, and chemicals for photograph processing at the landfill site (Mor et al. 2006; Moturi et al. 2004). Varying heavy metal concentrations have been reported in landfill leachate (Abu-Daabes et al. 2013; Baun and Christensen 2004). Generally, Zn content in the MSW is higher than heavy metals, such as Cu (Long et al. 2011). The current study suggests a relative higher Fe level. The heavy metal levels in this study were higher than levels from Iran, Sri Lanka, Brazil, and Pakistan (Aiman et al. 2016; de Oliveira et al. 2015; Wijesekara et al. 2014; Yao et al. 2014), while lower levels than those from India and Bangladesh (Nagarajan et al. 2012; Rikta et al. 2018) were observed. Cu, Pb, and Cr concentrations were lower than reported concentrations from Bosnia (Chakraborty and Kumar 2016).
Levels of heavy metals in borehole and well water collected from the communities around the Oti landfill site
The water samples were analyzed to access the Cd, Cr, Pb, Fe, Cu, and Zn concentration because they were characterized as unwanted heavy metals in drinking water (Nagarajan et al. 2012) and can infiltrate into the water system. The heavy metal concentration of the borehole and well water samples is given in Table 6.
The concentration of heavy metals in the landfill leachate was significantly higher than that in the groundwater samples. Along the groundwater flow direction, the concentration of heavy metals varied depending on the site of the groundwater, which can be described in terms of the quality and quantity of the leachate itself and the impact of the leachate with its affected groundwater samples. The changes in heavy metal associations in the groundwater can be attributed to the proportion of several heavy metals from the leachate, which contains high levels of Fe, Pb, and Cd and a relatively small proportion of Zn, Cr, and Cu. In addition to the large amount of heavy metal pollution originating from leachate, the degradation of a large amount of biodegradable material in the landfills also played a role (Liao et al. 2016). Biodegradation involves the use of a large amount of oxidant, for example, oxygen, in the aquifer matrix, which is in a relatively poorly ventilated groundwater table. This induces reducing conditions in the groundwater table, and the existing state of the affected groundwater by heavy metals is disturbed. This results in the heavy metals covering unusually high quantities of large particles, activated and non-activated colloids, and dissolved small particles. This can cause the heavy metals to be adsorbed on the pore of the aquifer. The mean level of Fe in the borehole samples ranged from 0.957 ± 0.319 mg/L at sampling site BH3 to 2.292 ± 1.756 mg/L at sampling site BH1, while that of the well water samples ranged from 0.732 ± 0.030 mg/L at sampling site W3 to 1.870 ± 0.532 mg/L at sampling site W1. The mean Pb concentrations in the borehole water samples ranged between 0.010 ± 0.006 mg/L at sampling site BH2 and 0.020 ± 0.001 mg/L at sampling site BH3, while that of the well water ranged between 0.076 ± 0.006 mg/L at sampling site W1 and 0.094 ± 0.017 mg/L at sampling site W4. The mean levels of Cd in the borehole water samples ranged between 0.002 ± 0.001 mg/L at sampling site BH3 and 0.033 ± 0.004 mg/L at sampling site BH1, while that of the well water ranged between 0.005 ± 0.001 mg/L at sampling site W3 and 0.020 ± 0.011 mg/L at sampling site W4. The mean levels of Zn in the well water samples varied between 0.068 ± 0.021 mg/L at sampling site W4 and 0.607 ± 0.154 mg/L at sampling site W1, while that of the borehole ranged from 0.057 ± 0.025 at sampling site BH3 to 0.237 ± 0.076 mg/L at sampling site BH1. The mean level of Cr in the borehole water samples ranged between 0.004 ± 0.003 mg/L at sampling site BH3 and 0.039 ± 0.023 mg/L at sampling site BH1, while that of the well water ranged from 0.052 ± 0.020 mg/L at sampling site W2 to 0.082 ± 0.033 mg/L at sampling site W1. The mean levels of Cu in the well water samples varied from 0.015 ± 0.002 mg/L at sampling site W4 to 0.040 ± 0.019 mg/L at sampling site W1, while that of the borehole water samples ranged between 0.014 ± 0.013 mg/L at sampling site BH2 and 0.247 ± 0.014 mg/L at sampling site BH1.
The levels of Zn, Cr, and Cu in most of the groundwater samples were below the WHO acceptable limit of 3.0, 0.05, and 2 mg/L for drinking water, respectively (WHO 2011). However, the groundwater samples collected from the Oti community area exhibited higher Fe, Pb, and Cd concentrations as compared to the WHO permitted value of 0.3, 0.01, and 0.003 mg/L for drinking water, respectively (WHO 2011). This study suggests that the main possible source of heavy metals in dump site and surrounding groundwater can be attributed to leachate percolation through the unlined waste dump site. The study area was surrounded by wastewater tributaries and dump sites, which may have an influence on high levels of reported heavy metals (Basharat and Rizvi 2011). High Pb levels in the groundwater could be due to the dumping of materials that contain lead batteries, pipes, and paints at the landfill site. Higher concentration of Fe can cause aesthetic (Lamikanra 1999) and hemochromatosis (Hopps 1972), especially when such water samples are used for drinking. Pb levels higher than 0.01 mg/L can cause neurological damage in young children and fetus when such water is used for drinking (WHO 2004). High levels of Pb can also cause brain damage and disruption of the nervous system (Bulut and Baysal 2006). The higher level of Cd can cause renal, arterial hypertension cramps, nausea, vomiting, and diarrhea when such water is used for drinking purposes (Lewis 1986). The concentrations of Cd, Pb, Zn, and Cr in the studied groundwater samples were comparable with earlier studies (Aiman et al. 2016; Chakraborty and Kumar 2016; Deshmukh and Aher 2016; Nagarajan et al. 2012; Wijesekara et al. 2014). In Pakistan, Pb concentrations were reported higher than the present study (Aiman et al. 2016). Zn levels recorded in well water sources close to landfill sites from the Tiruchirappalli District, India (Kanmani and Gandhimathi 2013), were higher than those in this study. Also, the concentrations of Fe in the present study were lower than the 23.0 mg/L reported by Chofqi et al. (2004) in well water samples collected from El Jadida, Morocco. Cr, Pb, and Cd levels were higher in this study than those recorded in Bosnia, while Zn, Cu, and Fe concentrations were lower than reported concentrations from Bosnia (Calo and Parise 2009). The concentrations of Cr, Zn, and Cu in groundwater of the main dump site were lower than those recorded in Nigeria, Egypt, India, and China landfills (El-Salam and Abu-Zuid 2015; Han et al. 2014).
Correlation analysis
Heavy metal distribution is closely related to organic matter and other pollutants in leachates (Wu et al. 2011). A correlation analysis was used to establish the relationships, as well as evaluating their common sources of the detected heavy metals in the underground water samples. The Pearson correlation analysis results are presented in Table 7a, b.
The Pearson correlation analysis revealed a strong positive correlation between Zn and Cd (r = 1.00, p < 0.01) in the borehole water samples (Table 7a). This suggests that Zn and Cd are derived from the same source. In the well water samples, a negative correlation detected between Zn and Pb (r = − 0.95, p < 0.05) suggests that both metals are influenced by different anthropogenic activities (Table 7b). The inter-elemental correlation between other variables was strong (r ≥± 0.70), but not significantly (p > 0.05). Poor correlation could be attributed to differences in mixed sources of origin and behavior of those heavy metals, as well as an anthropogenic influence (Sappa et al. 2014).
Potential sources of heavy metals
PCA has proved as an effective technique for offering vital information concerning heavy metal pathways and sources (Hou et al. 2013). The principal component was applied to the transformed data matrix to better comprehend the analyzed parameters, as well as decreasing the high dimensionality of the variable space. The Kaiser normalization was used to determine the number of components, for which components having eigenvalues > 1 were retained. Components loadings of < 0.5 show poor loadings, 0.5 designated moderate loadings, and > 0.5 denotes high loadings. The results of the principal components analysis in the boreholes and hand-dug water samples area are given in Table 8.
In this study, Bartlett’s test of sphericity and KMO were used to test the feasibility of PCA. The PCA result showed a smaller partial correlation between variables (KMO = 0.625) and a high dependence (p < 0.01). Generally, a p-value lower than 0.05 and a KMO value higher than 0.5 could be accepted as suitable for PCA. Two components with eigenvalues > 1 were found in the borehole water samples, explaining 97.99% of the total variance. The PC1 having 74.06% of the total variance was strongly correlated with Fe, Cd, Zn, and Cr. The presence of Zn, Cr, Cd, and Fe in the study area could be attributed to the anthropogenic origin, such as atmospheric depositions, domestic wastes, traffic sources municipal sewage, and industrial activities. High Cd level was mainly induced by industrial waste from paint (Frickel and Elliott 2008), and anthropogenic waste (Mann et al. 2002). However, Pb showed negative loading (− 0.597). The Cr association is predicted to affect by lithogenic effect, as well as natural input in the study area. Thus, PC1 could be contributed by anthropogenic and lithology component. The PC2 explained variance of 23.94% with significant loadings of Pd and Cu. The industrial waste and the discharge of leachate treatment plants were suggested as the major sources of Pb. Thus, PC2 could be contributed by the anthropogenic component.
In the hand-dug well water samples, two principal components were extracted, which accounted for 96.07% of the total variance. The PC1 with 77.46% of the total variance was positively related to Zn and Cu. High loadings of Cu could be due to Cu-containing agrochemicals (D’Adamo et al. 2014). Moreover, the presence of Zn could be due to the disposal of batteries, paints, and cosmetics in the landfill sites. PC2 with 18.61% of the total variance showed high loadings of Fe, Cr, and Cu. However, Cd and Pb revealed no strong correlations between the other heavy metals, suggesting their different chemical and physical process.
Human health risk assessment of the borehole and the well water samples
The human health risk assessment was evaluated using the USEPA risk assessment method (USEPA IRIS 2011). Table 9 shows a summary of HI on the health of consumers of water from borehole and well water samples in the Oti community.
Hazard quotients values for the detected heavy metals in the borehole samples were 0.026, 0.075, 0.022, 0.048, 0.318, and 0.005 for Fe, Cr, Cu, Pb, Cd, and Zn, respectively. In the hand-dug wells, the hazard quotients values were 0.022, 0.209, 0.007, 0.296, 0.312, and 0.014 for Fe, Cr, Cu, Pb, Cd, and Zn, respectively (Table 9). The hazard quotient values in all the groundwater samples were all < 1, and this suggests no adverse health effects even if the water is used for drinking. The HI values were 0.494 and 0.860 for the boreholes and hand-dug wells, respectively. The HI values recorded at sites BH1 and W4 were > 1, suggesting adverse health effects. The risk assessment suggested that Cd caused more pollution due to its high HI value. Exposure to high levels of Cd is associated with renal, arterial hypertension cramps, nausea, vomiting, and diarrhea (Ashraf 2012).
Because of the lack of carcinogenic SF of Fe, Cu, Zn, and Cd, only the slope factor for Pb is available in the literature. The RI values of Pb in the borehole and well water samples were recorded at 1.33 × 10−3 and 8.24 × 10−3, respectively. Moreover, the RI values of Cr in the borehole and well water samples were 0.12 and 0.34, respectively. The RI values of Pb and Cr in the borehole and well water samples were > 10−6, suggesting possible carcinogenic risk.
Pollution evaluation indices
The HPI, Cd, and HEI were evaluated using the average values of heavy metals in the borehole and well water samples. The pollution evaluation index results are given in Table 10.
The HPI values in the borehole samples ranged from 9.48 in BH3 to 37.68 in BH1. In the well water samples, the HPI values were between 9.11 and 21.39 in samples from W3 and W1 sites, respectively. The HPI results suggested that all the borehole and well water samples were less than the critical limit of 100 for drinking water (Prasad and Bose 2001). Moreover, the intensity of pollution progressively decreased from the landfill site to the downstream part of the groundwater samples. The HPI values from this study were lower than the HPI value of 518.55 and 250.77 reported by Ojekunle et al. (2016) and Bhuiyan et al. (2015), respectively. The degree of contamination was widely used as a reference method for estimating the extent of heavy metal pollution in water samples (Rubio et al. 2000). The degree of contamination values in the borehole and well water samples ranged from 10.93 to 26.79 and 57.70 to 70.90, respectively, as presented in Table 10. According to Backman et al. (1998), the degree of contamination of water samples can be classified as high, since all the samples recorded Cd values greater than 3. The range and mean value of Cd (0.25–14.80 and 4.12, respectively) reported by Bhuiyan et al. (2015) were lower than those in the hand-dug wells and boreholes samples at BH1 and BH3 sites. The HEI values ranged from 14.38 to 30.87 for borehole water samples. In the well water, the HEI values were between 64.15 and 74.93 (Table 10). The proposed heavy metal evaluation index criteria are classified as low (HEI < 10), medium (HEI = 10–20), and high (HEI > 20) (Edet and Offiong 2002). Based on the above classification, the heavy metal evaluation index results suggest that borehole samples at BH2 and BH3 sites fall within the medium zone, while groundwater samples at BH1, W1, W2, W3, and W4 sites fall within the high zone of heavy metal contamination. The HEI values ranging from 5.34 to 43.49 with a mean value of 24.75 reported by Bhuiyan et al. (2015) are comparable with those in this study.
Single-factor and a metal index in underground water
Using the water quality standards for WHO, the over-limit ratio of heavy metal and its pollution index was calculated in the underground water samples (WHO 2011). Over-limit ratio value > 0.1 suggests a threshold warning (Zhaoyong et al. 2015). The results, presented in Table 11, suggested that the over-limit ratio of Fe, Cd, Pb, and Cr in most of the water samples was above 0.1, rendering the water unacceptable for drinking.
According to metal index values, all the selected sites except BH3 were seriously polluted with heavy metals since the metal index values were greater than 6 as classified by Lyulko et al. (2001). The above observation suggests the impact of anthropogenic sources of the pollution load of groundwater in the Oti community.
Conclusion
The levels of Fe, Pb, and Cd had their average concentration in all the well water samples above the recommended maximum admissible limits approved by WHO for drinking water. In addition, Cr level at W1 site was above the maximum admissible limits. The level of Fe in all the borehole water samples was above the WHO permitted level. In addition, the levels of Pb at BH3 and Cd at BH1 and BH2 sites were also above the maximum limit. The health risk assessment of heavy metal in the borehole and well water samples recorded hazard quotient values that were < 1, suggesting an acceptable non-carcinogenic adverse health risk. However, hazard index values at BH1 and W4 sites were > 1, signifying adverse health effects. The HI value for Cd at BH1 sampling point was the most pollutants of non-carcinogenic concerns. The heavy metal pollution index values calculated for the borehole and well water samples were far less than the critical index limit of 100. However, the heavy metal evaluation index and the degree of contamination suggest contamination of all the well water samples. Moreover, the Cd values for all the boreholes were > 3 and the HEI value at site BH1 was also greater than 20, suggesting high heavy metal contamination at these sites. The MI result also computed to be > 6 suggests that the selected groundwater samples were seriously polluted with heavy metals. This study further predicted the impact of anthropogenic sources of the pollution load of groundwater in the Oti community. The WQI showed that all the well water samples and borehole samples at BH1 and BH2 sites fall under the poor water quality category, suggesting that water from BH1 and BH2 sites and all the well water samples may not be suitable for drinking. Multivariate analysis suggested anthropogenic and lithogenic factors as the possible source of water pollution of the heavy metal in the study area. Thus, multivariate statistical techniques could be a beneficial tool for an evaluation of the possible source of heavy metal pollution. The high levels of heavy metal suggest considerable pollution of groundwater by leachate percolation from the landfill site; therefore, we believe there is a need for new policies and methods, such as (1) the design of suitable management of selective collection and recycling of solid wastes, (2) collection and treatment systems at controlled landfill sites to reduce leachate impact on human health so that heavy metal and other pollution exposure situations can be prevented, (3) leachate collection and retardation of its movement via the design of new dumping sites with proper foundations, and finally, (4) open dumps must be closed and covered to minimize the penetration of precipitation into the landfill sites and leachate generation. The present observations can be of great significance in countries where there are inappropriate practices in solid waste management.
References
Abarikwu S, Iserhienrhien B, Badejo T (2013) Rutin-and selenium-attenuated cadmium-induced testicular pathophysiology in rats. Hum Exp Toxicol 32:395–406
Abu Qdais H (2010) Selection of landfill leachate management strategy using decision support system. J Solid Waste Technol Manag 36:246–257
Abu-Daabes M, Qdais HA, Alsyouri H (2013) Assessment of heavy metals and organics in municipal solid waste leachates from landfills with different ages in Jordan. J Environ Prot 4:344–352
Adamcová D et al (2017) Environmental assessment of the effects of a municipal landfill on the content and distribution of heavy metals in Tanacetum vulgare L. Chemosphere 185:1011–1018
Ahaneku IE, Adeoye PA (2014) Impact of pit latrines on groundwater quality of Fokoslum, Ibadan, southwestern Nigeria. Br J Appl Sci Technol 4:440–449
Aiman U, Mahmood A, Waheed S, Malik RN (2016) Enrichment, geo-accumulation and risk surveillance of toxic metals for different environmental compartments from Mehmood Booti dumping site, Lahore city, Pakistan. Chemosphere 144:2229–2237
Alimba C, Bakare A, Latunji C (2006) Municipal landfill leachates induced chromosome aberrations in rat bone marrow cells. Afr J Biotechnol 5:2053–2057
Alimba CG, Bakare AA, Aina OO (2012) Liver and kidney dysfunction in Wistar rats exposed to municipal landfill leachate. Resour Environ 2:150–163
Alloway BJ (2013) Sources of heavy metals and metalloids in soils. In: Alloway BJ (ed) Heavy metals in soils: trace metals and metalloids in soils and their bioavailability. Springer, Dordrecht, pp 11–50
APHA, WEF (2005) Standard methods for the examination of water and wastewater. American Public Health Association, American Water Works Association, and Water Environment Federation, Washington
Ashraf MW (2012) Levels of heavy metals in popular cigarette brands and exposure to these metals via smoking. Sci World J 2012:1–5
Ashworth SC (2005) Metal speciation and mobility as influenced by landfill disposal practices. In: Water encyclopedia. Wiley, New York City, United States
Assaad E et al (2007) Metal removal through synergic coagulation–flocculation using an optimized chitosan–montmorillonite system. Appl Clay Sci 37:258–274
Awasthi AK, Zeng X, Li J (2016) Environmental pollution of electronic waste recycling in India: a critical review. Environ Pollut 211:259–270
Azizi AB, Choy MY, Noor ZM, Noorlidah A (2015) Effect on heavy metals concentration from vermiconversion of agro-waste mixed with landfill leachate. Waste Manag 38:431–435
Backman B, Bodiš D, Lahermo P, Rapant S, Tarvainen T (1998) Application of a groundwater contamination index in Finland and Slovakia. Environ Geol 36:55–64
Basharat M, Rizvi SA (2011) Groundwater extraction and waste water disposal regulation. Is Lahore Aquifer at stake with as usual approach. In: Pakistan engg. congress; world water day, April 2011, pp 112–134
Baun DL, Christensen TH (2004) Speciation of heavy metals in landfill leachate: a review. Waste Manag Res 22:3–23
Bhuiyan MAH, Dampare SB, Islam M, Suzuki S (2015) Source apportionment and pollution evaluation of heavy metals in water and sediments of Buriganga River, Bangladesh, using multivariate analysis and pollution evaluation indices. Environ Monit Assess 187:1–21
Bulut Y, Baysal Z (2006) Removal of Pb(II) from wastewater using wheat bran. J Environ Manag 78:107–113
Calo F, Parise M (2009) Waste management and problems of groundwater pollution in karst environments in the context of a post-conflict scenario: the case of Mostar (Bosnia Herzegovina). Habitat Int 33:63–72
Chakraborty S, Kumar RN (2016) Assessment of groundwater quality at a MSW landfill site using standard and AHP based water quality index: a case study from Ranchi, Jharkhand, India. Environ Monit Assess 188:1–18
Chemlal R et al (2014) Combination of advanced oxidation and biological processes for the landfill leachate treatment. Ecol Eng 73:281–289
Cheng Z, Man YB, Nie XP, Wong MH (2013) Trophic relationships and health risk assessments of trace metals in the aquaculture pond ecosystem of Pearl River Delta, China. Chemosphere 90:2142–2148
Chofqi A, Younsi A, Lhadi EK, Mania J, Mudry J, Veron A (2004) Environmental impact of an urban landfill on a coastal aquifer (El Jadida, Morocco). J Afr Earth Sci 39:509–516
D’Adamo R, Specchiulli A, Cassin D, Botter M, Zonta R, Fabbrocini A (2014) The effect of floods on sediment contamination in a microtidal coastal lagoon: the Lagoon of Lesina, Italy. Arch Environ Contam Toxicol 67:297–309
de Oliveira DM, Dos Santos MJS, Sousa Filho HR, Cardoso LAM, de Souza CT, Bezerra MA, Souza AS (2015) Multivariate exploratory analysis of metals and phosphorus concentrations of leachates collected monthly from a municipal sanitary landfill. Bull Environ Contam Toxicol 95:97–101
Demim S, Drouiche N, Aouabed A, Semsari S (2013) CCD study on the ecophysiological effects of heavy metals on Lemna gibba. Ecol Eng 57:302–313
Deshmukh KK, Aher SP (2016) Assessment of the impact of municipal solid waste on groundwater quality near the Sangamner City using GIS approach. Water Resour Manag 30:2425–2443
Edet A, Offiong O (2002) Evaluation of water quality pollution indices for heavy metal contamination monitoring. A study case from Akpabuyo-Odukpani area, Lower Cross River Basin (southeastern Nigeria). GeoJournal 57:295–304
El-Salam MMA, Abu-Zuid GI (2015) Impact of landfill leachate on the groundwater quality: a case study in Egypt. J Adv Res 6:579–586
EPA-Ghana (2001) Status of sewage treatment plants: internal monitoring report. EPA, Accra
Farombi E, Akintunde J, Nzute N, Adedara I, Arojojoye O (2012) Municipal landfill leachate induces hepatotoxicity and oxidative stress in rats. Toxicol Ind Health 28:532–541
Foul AA, Aziz HA, Isa MH, Hung Y-T (2009) Primary treatment of anaerobic landfill leachate using activated carbon and limestone: batch and column studies. Int J Environ Waste Manag 4:282–298
Frickel S, Elliott JR (2008) Tracking industrial land use conversions a new approach for studying relict waste and urban development. Organ Environ 21:128–147
Gonzalez-Valencia R, Magana-Rodriguez F, Cristóbal J, Thalasso F (2016) Hotspot detection and spatial distribution of methane emissions from landfills by a surface probe method. Waste Manag 55:299–305
Grugnaletti M, Pantini S, Verginelli I, Lombardi F (2016) An easy-to-use tool for the evaluation of leachate production at landfill sites. Waste Manag 55:204–219
Guan S, Yu S, Zhong S (2014) Leak marking scheme for construction quality testing of geomembrane liners in landfills. Int J Environ Res 8:447–452
Guo XJ, Li Q, Jiang JY, Dai BL (2014) Investigating spectral characteristics and spatial variability of dissolved organic matter leached from wetland in semi-a rid region to differentiate its sources and fate. CLEAN Soil Air Water 42:1076–1082
Han D, Tong X, Currell MJ, Cao G, Jin M, Tong C (2014) Evaluation of the impact of an uncontrolled landfill on surrounding groundwater quality, Zhoukou, China. J Geochem Explor 136:24–39
Hopps HC (1972) Ecology of disease in relation to environmental trace elements—particularly iron. Geol Soc Spec Pap 140:1–8
Horton RK (1965) An index number system for rating water quality. J Water Pollut Control Fed 37:300–306
Hou D, He J, Lü C, Ren L, Fan Q, Wang J, Xie Z (2013) Distribution characteristics and potential ecological risk assessment of heavy metals (Cu, Pb, Zn, Cd) in water and sediments from Lake Dalinouer, China. Ecotoxicol Environ Saf 93:135–144
Hu X, Zhang Y, Ding Z, Wang T, Lian H, Sun Y, Wu J (2012) Bioaccessibility and health risk of arsenic and heavy metals (Cd Co, Cr, Cu, Ni, Pb, Zn and Mn) in TSP and PM2.5 in Nanjing. China Atmos Environ 57:146–152
Huang H et al (2011) Quantitative evaluation of heavy metals’ pollution hazards in liquefaction residues of sewage sludge. Bioresour Technol 102:10346–10351
Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN (2014) Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol 7:60–72
Kanmani S, Gandhimathi R (2013) Investigation of physicochemical characteristics and heavy metal distribution profile in groundwater system around the open dump site. Appl Water Sci 3:387–399
Kjeldsen P, Barlaz MA, Rooker AP, Baun A, Ledin A, Christensen TH (2002) Present and long-term composition of MSW landfill leachate: a review. Crit Rev Environ Sci Technol 32:297–336
Kucuksezgin F, Kontas A, Altay O, Uluturhan E, Darılmaz E (2006) Assessment of marine pollution in Izmir Bay: nutrient, heavy metal and total hydrocarbon concentrations. Environ Int 32:41–51
Kumar D, Alappat BJ (2005) Analysis of leachate pollution index and formulation of sub-leachate pollution indices. Waste Manag Res 23:230–239
Lamikanra A (1999) Essential microbiology for students and practitioner of pharmacy, medicine and microbiology, 2nd edn. Amkra Books, Lagos, p 406
Landaburu-Aguirre J, García V, Pongrácz E, Keiski RL (2009) The removal of zinc from synthetic wastewaters by micellar-enhanced ultrafiltration: statistical design of experiments. Desalination 240:262–269
Lee J-S, Chon H-T, Kim H-T (2005) Human risk assessment of As, Cd, Cu and Zn in the abandoned metal mine site. Environ Geochem Health 27:185–191
Lewis SR (1986) Hazardous chemicals desk reference, 6th edn. Wiley, Hoboken
Li Z, Ma Z, van der Kuijp TJ, Yuan Z, Huang L (2014) A review of soil heavy metal pollution from mines in China: pollution and health risk assessment. Sci Total Environ 468:843–853
Liao P, Yuan S, Wang D (2016) Impact of redox reactions on colloid transport in saturated porous media: an example of ferrihydrite colloids transport in the presence of sulfide. Environ Sci Technol 50:10968–10977
Lim HS, Lee JS, Chon HT, Sager M (2008) Heavy metal contamination and health risk assessment in the vicinity of the abandoned Songcheon Au–Ag mine in Korea. J Geochem Explor 96:223–230
Long Y-Y, Shen D-S, Wang H-T, Lu W-J, Zhao Y (2011) Heavy metal source analysis in municipal solid waste (MSW): case study on Cu and Zn. J Hazard Mater 186:1082–1087
Longe EO, Balogun MR (2010) Groundwater quality assessment near a municipal landfill, Lagos, Nigeria. Res J Appl Sci Eng Technol 2:39–44
Lyulko I, Ambalova T, Vasiljeva T (2001) To integrated water quality assessment in Latvia. In: MTM (monitoring tailor-made) III, proceedings of international workshop on information for sustainable water management, Netherlands, pp 449–452
Ma W, Tai L, Qiao Z, Zhong L, Wang Z, Fu K, Chen G (2018) Contamination source apportionment and health risk assessment of heavy metals in soil around municipal solid waste incinerator: a case study in North China. Sci Total Environ 631:348–357
MacDonald AM, Calow RC (2009) Developing groundwater for secure rural water supplies in Africa. Desalination 248:546–556
Mann S, Rate A, Gilkes R (2002) Cadmium accumulation in agricultural soils in Western Australia. Water Air Soil Pollut 141:281–297
Mavakala BK et al (2016) Leachates draining from controlled municipal solid waste landfill: detailed geochemical characterization and toxicity tests. Waste Manag 55:238–248
Mohammed S (2004) Urbanization and water resources vulnerability in the Kumasi Metropolitan area. Linkoping University, Ghana
Mohan SV, Nithila P, Reddy SJ (1996) Estimation of heavy metals in drinking water and development of heavy metal pollution index. J Environ Sci Health A 31:283–289
Mojiri A, Aziz HA, Zaman NQ, Aziz SQ, Zahed MA (2016) Metals removal from municipal landfill leachate and wastewater using adsorbents combined with biological method. Desalination Water Treat 57:2819–2833
Mor S, Ravindra K, Dahiya R, Chandra A (2006) Leachate characterization and assessment of groundwater pollution near municipal solid waste landfill site. Environ Monit Assess 118:435–456
Moturi M, Rawat M, Subramanian V (2004) Distribution and fractionation of heavy metals in solid waste from selected sites in the industrial belt of Delhi, India. Environ Monit Assess 95:183–199
Nagajyoti PC, Lee KD, Sreekanth T (2010) Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett 8:199–216
Nagarajan R, Thirumalaisamy S, Lakshumanan E (2012) Impact of leachate on groundwater pollution due to non-engineered municipal solid waste landfill sites of erode city, Tamil Nadu, India. Iran J Environ Health Sci Eng 9:1–12
Nartey VK, Hayford EK, Ametsi SK (2012) Assessment of the impact of solid waste dumpsites on some surface water systems in the Accra Metropolitan area, Ghana. J Water Resour Prot 4:605–615
Naveen B, Mahapatra DM, Sitharam T, Sivapullaiah P, Ramachandra T (2017) Physico-chemical and biological characterization of urban municipal landfill leachate. Environ Pollut 220:1–12
Nyame FK, Tigme J, Kutu JM, Armah TK (2012) Environmental implications of the discharge of municipal landfill leachate into the Densu River and surrounding Ramsar wetland in the Accra Metropolis, Ghana. J Water Resour Prot 4:622–633
Ojekunle OZ, Ojekunle OV, Adeyemi AA, Taiwo AG, Sangowusi OR, Taiwo AM, Adekitan AA (2016) Evaluation of surface water quality indices and ecological risk assessment for heavy metals in scrap yard neighbourhood. SpringerPlus 5:1–16
Osei J et al (2011) The impact of Oblogo landfill site in Accra-Ghana on the surrounding environment. Res J Environ Earth Sci 3:633–636
Oyeku O, Eludoyin A (2010) Heavy metal contamination of groundwater resources in a Nigerian urban settlement. Afr J Environ Sci Technol 4:201–214
Pantini S, Verginelli I, Lombardi F (2014) A new screening model for leachate production assessment at landfill sites. Int J Environ Sci Technol 11:1503–1516
Peters TA (1998) Purification of landfill leachate with reverse osmosis and nanofiltration. Desalination 119:289–293
Pohland FG, Harper SR (1987) Retrospective evaluation of the effects of selected industrial wastes on municipal-solid-waste stabilization in simulated landfills. Hazardous Waste Engineering Research Laboratory, Office of Research and Development, Cincinnati
Prasad B, Bose J (2001) Evaluation of the heavy metal pollution index for surface and spring water near a limestone mining area of the lower Himalayas. Environ Geol 41:183–188
Renou S, Givaudan J, Poulain S, Dirassouyan F, Moulin P (2008) Landfill leachate treatment: review and opportunity. J Hazard Mater 150:468–493
Rikta S, Tareq SM, Uddin MK (2018) Toxic metals (Ni2+, Pb2+, Hg2+) binding affinity of dissolved organic matter (DOM) derived from different ages municipal landfill leachate. Appl Water Sci 8:1–8
Rubio B, Nombela M, Vilas F (2000) Geochemistry of major and trace elements in sediments of the Ria de Vigo (NW Spain): an assessment of metal pollution. Mar Pollut Bull 40:968–980
Samadder S, Prabhakar R, Khan D, Kishan D, Chauhan M (2017) Analysis of the contaminants released from municipal solid waste landfill site: a case study. Sci Total Environ 580:593–601
Sappa G, Ergul S, Ferranti F (2014) Geochemical modeling and multivariate statistical evaluation of trace elements in arsenic contaminated groundwater systems of Viterbo Area, (Central Italy). SpringerPlus 3:1–19
Schrapp K, Al–Mutairi N (2010) Associated health effects among residences near Jeleeb Al-Shuyoukh landfill. Am J Environ Sci 6:184–190
Siriwong W (2006) Organophosphate pesticide residues in aquatic ecosystem and health risk assessment of local agriculture community. Chulalongkorn University, Bangkok
Smith AH, Lingas EO, Rahman M (2000) Contamination of drinking-water by arsenic in Bangladesh: a public health emergency. Bull WHO 78:1093–1103
Souiri M et al (2009) Escherichia coli-functionalized magnetic nanobeads as an ultrasensitive biosensor for heavy metals. Procedia Chem 1:1027–1030
Tamasi G, Cini R (2004) Heavy metals in drinking waters from Mount Amiata (Tuscany, Italy). Possible risks from arsenic for public health in the Province of Siena. Sci Total Environ 327:41–51
Tolaymat T, Kremer F, Carson D, Davis-Hoover W (2004) Monitoring approaches for landfill bioreactors. National Risk Management Research Laboratory, Office of Research and Development, Ohio
USEPA (1989) Risk assessment guidance for Superfund. Human health evaluation manual, (part A), vol 1. Office of emergency and remedial response, Washington
USEPA (2011) Exposure factors handbook. National Center for Environmental Assessment. United State Environmental Protection Agency, Washington
USEPA IRIS (2011) Integrated risk information system. Environmental Protection Agency Region I. United State Environmental Protection Agency, Washington, p 20460
WHO (2004) Guidelines for drinking-water quality: recommendations, vol 1. World Health Organization, Geneva
WHO (2011) Guidelines for drinking-water quality, 4th edn. World health organization, Geneva
Wijesekara S, Mayakaduwa SS, Siriwardana A, de Silva N, Basnayake B, Kawamoto K, Vithanage M (2014) Fate and transport of pollutants through a municipal solid waste landfill leachate in Sri Lanka. Environ Earth Sci 72:1707–1719
Wittsiepe J, Feldt T, Till H, Burchard G, Wilhelm M, Fobil JN (2017) Pilot study on the internal exposure to heavy metals of informal-level electronic waste workers in Agbogbloshie, Accra, Ghana. Environ Sci Pollut Res 24:3097–3107
Wongsasuluk P, Chotpantarat S, Siriwong W, Robson M (2014) Heavy metal contamination and human health risk assessment in drinking water from shallow groundwater wells in an agricultural area in Ubon Ratchathani province, Thailand. Environ Geochem Health 36:169–182
Wu J, Zhang H, He P-J, Shao L-M (2011) Insight into the heavy metal binding potential of dissolved organic matter in MSW leachate using EEM quenching combined with PARAFAC analysis. Water Res 45:1711–1719
Yao J, Kong Q, Li W, Zhu H, Shen D (2014) Effect of leachate recirculation on the migration of copper and zinc in municipal solid waste and municipal solid waste incineration bottom ash co-disposed landfill. J Mater Cycles Waste Manag 16:775–783
Zhaoyong Z, Abuduwaili J, Fengqing J (2015) Heavy metal contamination, sources, and pollution assessment of surface water in the Tianshan Mountains of China. Environ Monit Assess 187:1–13
Ziyang L, Youcai Z, Tao Y, Yu S, Huili C, Nanwen Z, Renhua H (2009) Natural attenuation and characterization of contaminants composition in landfill leachate under different disposing ages. Sci Total Environ 407:3385–3391
Acknowledgements
The authors are very grateful to the National Council for Tertiary Education (NTCE), Ghana, for a research Grant under the Teaching and Learning Innovation Fund (TALIFKNUSTR/3/005/2005). The authors are also grateful to J. Stanley-Owusu and Company Limited for granting access to the Oti landfill site.
Author information
Authors and Affiliations
Contributions
All the authors contributed equally to the preparation of this manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Boateng, T.K., Opoku, F. & Akoto, O. Heavy metal contamination assessment of groundwater quality: a case study of Oti landfill site, Kumasi. Appl Water Sci 9, 33 (2019). https://doi.org/10.1007/s13201-019-0915-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-019-0915-y