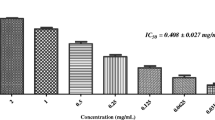
Abstract
Azadirachta indica, Emblica officinalis, Syzygium cumini and Terminalia bellirica are common in Indian system of traditional medicine for the prevention of diabetes and its complications. The aim of the present study was to comprehensively and comparatively investigate the antiglycation potential of these plant extracts at multiple stages and their possible protective effect against glycated albumin mediated toxicity to erythrocytes. Antiglycation activities of these plant extracts was measured by co-incubation of plant extract with bovine serum albumin-fructose glycation model. The multistage glycation markers- fructosamines (early stage), protein carbonyls (intermediate stage) and AGEs (late stage) are investigated along with measurement of thiols and β aggregation of albumin using amyloid-specific dyes–Congo red and Th T. Protection of erythrocytes from glycated albumin induced toxicity by these plant extracts was assessed by measuring erythrocytes hemolysis, lipid peroxidation, reduced glutathione and intracellular antioxidant capacity. Total phenolics, reducing power and antioxidant activities of the plant extracts were also measured. In vitro glycation assays showed that plant extracts exerted site specific inhibitory effects at multiple stages, with T. bellirica showing maximum attenuation. In erythrocytes, along with the retardation of glycated albumin induced hemolysis and lipid-peroxidation, T. bellirica considerably maintained cellular antioxidant potential. Significant positive correlations were observed between erythrocyte protection parameters with total phenolics. These plant extracts especially T. bellirica prevents glycation induced albumin modifications and subsequent toxicity to erythrocytes which might offer additional protection against diabetic vascular complications.






Similar content being viewed by others
Abbreviations
- ABTS:
-
2, 2′azino-bis (3-ethylbenzothizoline-6-sulphonic acid)
- AGEs:
-
Advanced glycation end products
- BHT:
-
Butylated hydroxyl toluene
- BSA:
-
Bovine serum albumin
- DNPH:
-
di-nitro phenyl hydrazine
- DPPH:
-
2,2′-diphenyl-1-picrylhydrazyl
- DTNB:
-
5, 5-Dithiobis- 2-nitrobenzoic acid
- FC:
-
Folin–Ciocalteu
- GAE:
-
Gallic acid equivalents
- LPO:
-
Lipid peroxidation
- MDA:
-
Malondialdehyde
- OS:
-
Oxidative stress
- RP:
-
Reducing power
- Th. T:
-
Thioflavin T
References
Asgary S, Naderi GH, Askari N (2005) Protective effect of flavonoids against red blood cell hemolysis by free radicals. Exp Clin Cardiol 10:88–90
Baker JR, Metcalf PA, Johnson RN, Newman D, Rietz P (1985) Use of protein-based standards in automated colorimetric determinations of fructosamine in serum. Clin Chem 31:1550–1554
Barnaby OS, Cerny RL, Clarke W, Hage DS (2011) Comparison of modification sites formed on human serum albumin at various stages of glycation. Clin Chim Acta 412:277–285
Benzie IFF, Strain JJ (1998) Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol 299:15–27
Bouma B, Kroon-Batenburg LM, Wu YP, Brünjes B, Posthuma G, Kranenburg O et al (2003) Glycation induces formation of amyloid cross-β structure in albumin. J Biol Chem 278:41810–41819
Bourdon E, Loreau N, Blache D (1999) Glucose and free radicals impair the antioxidant properties of serum albumin. FASEB J 13:233–244
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol 28:25–30
Brownlee M (2001) Biochemistry and molecular cell biology of diabetic complications. Nature 414:813–820
Candiloros H, Muller S, Ziegler O, Donner M, Drouin P (1996) Role of albumin glycation on the erythrocyte aggregation: an in vitro study. Diabet Med 13:646–650
Cohen MP (2003) Intervention strategies to prevent pathogenetic effects of glycated albumin. Arch Biochem Biophys 419:25–30
Dean PDG, Johnson WS, Middle FA (eds) (1985) Affinity chromatography: a practical approach. IRL Press, Oxford, p 133
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Goh SY, Cooper ME (2008) The role of advanced glycation end products in progression and complications of diabetes. J Clin Endocrinol Metab 93:1143–11452
Gutierrez RM, Gómez YG, Guzman MD (2011) Attenuation of nonenzymatic glycation, hyperglycemia, and hyperlipidemia in streptozotocin-induced diabetic rats by chloroform leaf extract of Azadirachta indica. Pharmacogn Mag 7:254–259
Hudson SA, Ecroyd H, Kee TW, Carver JA (2009) The thioflavin T fluorescence assay for amyloid fibril detection can be biased by the presence of exogenous compounds. FEBS J 276:5960–5972
Kasabri V, Flatt PR, Abdel-Wahab YHA (2010) Terminalia bellirica stimulates the secretion and action of insulin and inhibits starch digestion and protein glycation in vitro. Br J Nutr 103:212–217
Klunk WE, Jacob RF, Mason RP (1999) Quantifying amyloid by congo red spectral shift assay. Methods Enzymol 309:285–305
Konyalioglu S, Karamenderes C (2005) The protective effects of Achillea L. species native in Turkey against H2O2-induced oxidative damage in human erythrocytes and leucocytes. J Ethnopharmacol 102:221–227
LeVine H (1999) Quantification of β -sheet amyloid fibril structures with thioflavin T. Methods Enzymol 309:274–284
Lim YY, Quah EPL (2007) Antioxidative tyrosinase inhibiting and antibacterial activities of leaf extracts from medicinal ferns. Food Chem 103:734–740
Luize PS, Tiuman TS, Morello LG, Maza PK, Ueda-Nakamura T, Dias Filho BP et al (2005) Effects of medicinal plant extracts on growth of leishmania (l.) amazonensis and trypanosoma cruzi. Braz J Pharm Sci 41:85–94
Martín-Gallán P, Carrascosa A, Gussinyé M, Domínguez C (2003) Biomarkers of diabetes-associated oxidative stress and antioxidant status in young diabetic patients with or without subclinical complications. Free Radic Biol Med 34:1563–1574
McPherson JD, Shilton BH, Walton DJ (1988) Role of fructose in glycation and cross-linking of proteins. Biochemistry 27:1901–1907
Modak M, Dixit P, Londhe J, Ghaskadbi S, Thomas P, Devasagaya A (2007) Indian herbs and herbal drugs used for the treatment of diabetes. J Clin Biochem Nutr 40:163–173
Mossine VV, Linetsky M, Glinsky GV, Ortwerth BJ, Feather MS (1999) Superoxide free radical generation by Amadori compounds: the role of acyclic forms and metal ions. Chem Res Toxicol 12:230–236
Mukherjee PK, Maiti K, Mukherjee K, Houghton PJ (2006) Leads from Indian medicinal plants with hypoglycemic potentials. J Ethnopharmacol 106:1–28
Nampoothiri SV, Prathapan A, Cherian OL, Raghu KG, Venugopalan VV, Sundaresan A (2011) In vitro antioxidant and inhibitory potential of Terminalia bellirica and Emblica officinalis fruits against ldl oxidation and key enzymes linked to type 2 diabetes. Food Chem Toxicol 49:125–131
Oyaizu M (1986) Studies on products of browning reaction: antioxidative activity of product of browning reaction prepared from glucosamine. Jpn J Nutr 44:307–315
Paiva-Martins F, Fernandes J, Rocha S, Nascimento H, Vitorino R, Amado F et al (2009) Effects of olive oil polyphenols on erythrocyte oxidative damage. Mol Nutr Food Res 53:609–616
Peng X, Cheng KW, Ma J, Chen B, Ho CT, Lo C et al (2008) Cinnamon bark proanthocyanidins as reactive carbonyl scavengers to prevent the formation of advanced glycation endproducts. J Agric Food Chem 56:1907–1911
Placer ZA, Cushman LL, Johnson BC (1966) Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal Biochem 16:359–364
Rahbar S, Figarola JL (2003) Novel inhibitors of advanced glycation endproducts. Arch Biochem Biophys 419:63–79
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved abts radical cation decolorization assay. Free Radic Biol Med 26:1231–1237
Rondeau P, Bourdon E (2011) The glycation of albumin: structural and functional impacts. Biochimie 93:645–658
Saraswat M, Reddy PY, Muthenna P, Reddy GB (2009) Prevention of non-enzymic glycation of proteins by dietary agents: prospects for alleviating diabetic complications. Br J Nutr 101:1714–1721
Sattarahmady N, Moosavi-Movahedi AA, Ahmad F, Hakimelahi GH, Habibi-Rezaei M, Saboury AA et al (2007) Formation of the molten globule-like state during prolonged glycation of human serum albumin. Biochim Biophys Acta Gen Subj 1770:933–942
Tupe RS, Agte VV (2010) Role of zinc along with ascorbic acid and folic acid during long-term in vitro albumin glycation. Br J Nutr 103:370–377
Uchida K, Kanematsu M, Sakai K, Matsuda T, Hattori N, Mizuno Y et al (1998) Protein-bound acrolein: potential markers for oxidative stress. Proc Natl Acad Sci U S A 95:4882–4887
Vetter SW, Indurthi VSK (2011) Moderate glycation of serum albumin affects folding, stability, and ligand binding. Clin Chim Acta 412:2105–2116
Waczulíkova I, Sikurová L, Cársky J, Strbová L, Krahulec B (2000) Decreased fluidity of isolated erythrocyte membranes in type 1 and type 2 diabetes. The effect of resorcylidene aminoguanidine. Gen Physiol Biophys 19:381–392
Wautier JL, Wautier MP, Chappey O, Zoukourian C, Guillausseau PJ, Capron L (1996) Diabetic erythrocytes bearing advanced glycation end products induce vascular dysfunctions. Clin Hemorheol 16:661–667
Wu CH, Yeh CT, Shih PH, Yen GC (2010) Dietary phenolic acids attenuate multiple stages of protein glycation and high-glucose-stimulated proinflammatory IL-1beta activation by interfering with chromatin remodeling and transcription in monocytes. Mol Nutr Food Res 54:S127–S140
Wu CH, Huang SM, Lin JA, Yen GC (2011) Inhibition of advanced glycation end product formation by foodstuffs. Food Funct 2:224–234
Yamagishi S, Matsui T (2010) Advanced glycation end products, oxidative stress and diabetic nephropathy. Oxidative Med Cell Longev 3:101–108
Acknowledgments
The financial assistance from Department of Science and Technology, New Delhi, India is gratefully acknowledged. The authors wish to record their sincere thanks to Principal G. D. Sharma and late Professor R. M. Kothari, for encouragement and helpful suggestions for the research work. We acknowledge Dr. S. Gaikwad (Biochemical Sciences Division, National Chemical Laboratory, and Pune) for help in spectroflurometric analysis. Part of this work was presented at the 43rd National Conference of Nutrition Society of India’s 50th meeting held at National Institute of Nutrition, Hyderabad, India during 11 to12 Nov. 2011.
Author disclosure statement
No competing financial interests exist for any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tupe, R.S., Sankhe, N.M., Shaikh, S.A. et al. Aqueous extract of some indigenous medicinal plants inhibits glycation at multiple stages and protects erythrocytes from oxidative damage–an in vitro study. J Food Sci Technol 52, 1911–1923 (2015). https://doi.org/10.1007/s13197-013-1211-8
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-013-1211-8