Abstract
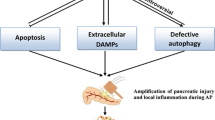
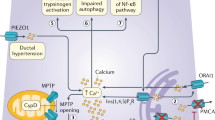
The precise mechanisms involved in the pathophysiology of acute pancreatitis (AP) are still far from clear. Several earlier studies have focused mainly on pancreatic enzyme activation as the key intracellular perturbation in the pancreatic acinar cells. For decades, the trypsin-centered hypothesis has remained the focus of the intra-acinar events in acute pancreatitis. Recent advances in basic science research have lead to the better understanding of various other mechanisms such as oxidative and endoplasmic stress, impaired autophagy, mitochondrial dysfunction, etc. in causing acinar cell injury. Despite all efforts, the clinical outcome of patients with AP has not changed significantly over the years. This suggests that the knowledge of the critical molecular pathways in the pathophysiology of AP is still limited. The mechanisms through which the acinar cell injury leads to local and systemic inflammation are not well understood. The role of inflammatory markers and immune system activation is an area of much relevance from the point of view of finding a target for therapeutic intervention. Some data are available from experimental animal models but not much is known in human pancreatitis. This review intends to highlight the current understanding in this area.






Similar content being viewed by others
References
Isenmann R, Beger HG. Natural history of acute pancreatitis and the role of infection. Best Pract Res Clin Gastroenterol. 1999;13:291–301.
Forsmark CE, Baillie J. AGA Institute technical review on acute pancreatitis. Gastroenterology. 2007;132:2022–44.
Chiari H. About the digestion of the human pancreas (in German). ZeitschriftfuHeilkunde. 1896;17:69–96.
Lerch MM, Gorelick FS. Early trypsinogen activation in acute pancreatitis. Med Clin North Am. 2000;84:549–63.
Saluja AK, Lerch MM, Phillips PA, Dudeja V. Why does pancreatic overstimulation cause pancreatitis? Annu Rev Physiol. 2007;69:249–69.
Cosen-Binker LI, Gaisano HY. Recent insights into the cellular mechanisms of acute pancreatitis. Can J Gastroenterol. 2007;21:19–24.
Hashimoto D, Ohmuraya M, Hirota M, et al. Involvement of autophagy in trypsinogen activation within the pancreatic acinar cells. J Cell Biol. 2008;181:1065–72.
Sharma M, Banerjee D, Garg PK. Characterization of newer subgroups of fulminant and subfulminant pancreatitis associated with a high early mortality. Am J Gastroenterol. 2007;102:2688–95.
Gaiser S, Daniluk J, Liu Y, et al. Intracellular activation of trypsinogen in transgenic mice induces acute but not chronic pancreatitis. Gut. 2011;60:1379–88.
Dawra R, Sah RP, Dudeja V, et al. Intra-acinar trypsinogen activation mediates early stages of pancreatic injury but not inflammation in mice with acute pancreatitis. Gastroenterology. 2011;141:2210–7. e2.
Criddle DN, Gerasimenko JV, Baumgartner HK, et al. Calcium signalling and pancreatic cell death: apoptosis or necrosis? Cell Death Differ. 2007;14:1285–94.
Petersen OH. Ca2+ induced pancreatic cell death: roles of the endoplasmic reticulum, zymogen granules, lysosomes and endosomes. J Gastroenterol Hepatol. 2008;23 Suppl 1:S31–6.
Sutton R, Criddle D, Raraty MG, Tepikin A, Neoptolemos JP, Petersen OH. Signal transduction, calcium and acute pancreatitis. Pancreatology. 2003;3:497–505.
Husain SZ, Prasad P, Grant WM, Kolodecik TR, Nathanson MH, Gorelick FS. The ryanodine receptor mediates early zymogen activation in pancreatitis. Proc Natl Acad Sci U S A. 2005;102:14386–91.
Cahalan MD. STIMulating store-operated Ca(2+) entry. Nat Cell Biol. 2009;11:669–77.
Lee KP, Yuan JP, Hong JH, So I, Worley PF, Muallem S. An endoplasmic reticulum/plasma membrane junction: STIM1/Orai1/TRPCs. FEBS Lett. 2010;584:2022–27.
Orabi AI, Shah AU, Ahmad MU, et al. Dantrolene mitigates caerulein-induced pancreatitis in vivo in mice. Am J Physiol Gastrointest Liver Physiol. 2010;299:G196–204.
Kim MS, Lee KP, Yang D, et al. Genetic and pharmacologic inhibitionof the Ca2+ influx channel TRPC3 protects secretory epithelia from Ca2+ dependent toxicity. Gastroenterology. 2011;140:2107–15.
Calcraft PJ, Ruas M, Pan Z, et al. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature. 2009;459:596–600.
Ogunbayo OA, Zhu Y, Rossi D, et al. Cyclic adenosine diphosphate ribose activates ryanodine receptors, whereas NAADP activates two-pore domain channels. J Biol Chem. 2011;286:9136–40.
Voronina SG, Barrow SL, Simpson AW, et al. Dynamic changes in cytosolic and mitochondrial ATP levels in pancreatic acinar cells. Gastroenterology. 2010;138:1976–87.
Saluja AK, Donovan EA, Yamanaka K, Yamaguchi Y, Hofbauer B, Steer ML. Caerulein induced in vitro activation of trypsinogen in rat pancreatic aciniis mediated by cathepsin B. Gastroenterology. 1997;113:304–10.
Meister T, Niehues R, Hahn D, et al. Missorting of cathepsin B into the secretory compartment of CI-MPR/IGFII-deficient mice does not induce spontaneous trypsinogen activation but leads to enhanced trypsin activity during experimental pancreatitis without affecting disease severity. J Physiol Pharmacol. 2010;61:565–75.
Behrendorff N, Floetenmeyer M, Schwiening C, Thorn P. Protons released during pancreatic acinar cell secretion acidify the lumen and contribute to pancreatitis in mice. Gastroenterology. 2010;139:1711–20.
Waterford SD, Kolodecik TR, Thrower EC, Gorelick FS. Vacuolar ATPase regulates zymogen activation in pancreatic acini. J Biol Chem. 2005;280:5430–4.
Reed AM, Husain SZ, Thrower E, et al. Low extracellular pH induces damage in the pancreatic acinar cell by enhancing calcium signaling. J Biol Chem. 2010;286:1919–26.
Grasso D, Ropolo A, Lo Re A, et al. Zymophagy, a novel selective autophagy pathway mediated by VMP1-USP9x-p62, prevents pancreatic cell death. J Biol Chem. 2011;286:8308–24.
Mareninova OA, Hermann K, French SW, et al. Impaired autophagic flux mediates acinar cell vacuole formation and trypsinogen activation in rodent models of acute pancreatitis. J Clin Invest. 2009;119:3340–55.
Jacob TG, Vipin IS, Roy TS, Garg PK. Electron-microscopic evidence of mitochondriae containing macroautophagy in experimental acute pancreatitis: implications for cell death. Pancreatology. 2014;14:433–5.
Sanfey H, Bulkley GB, Cameron JL. The role of oxygen-derived free radicals in the pathogenesis of acute pancreatitis. Ann Surg. 1984;200:405–13.
Sweiry JH, Mann GE. Role of oxidative stress in the pathogenesis of acute pancreatitis. Scand J Gastroenterol Suppl. 1996;219:10–5.
Tsai K, Wang SS, Chen TS, et al. Oxidative stress: an important phenomenon with pathogenetic significance in the progression of acute pancreatitis. Gut. 1998;42:850–5.
Chan YC, Leung PS. Angiotensin-II type 1 receptor-dependent nuclear factor-kβ activation mediated proinflammatory actions in a rat model of obstructive acute pancreatitis. J Pharmacol Exp Ther. 2007;323:10–8.
Escobar J, Pereda J, Lopez-Rodas G, Sastre J. Redox signaling and histone acetylation in acute pancreatitis. Free Radic Biol Med. 2012;52:819–37.
Ushio-Fukai M. Compartmentalization of redox signalling through NADPH oxidase-derived ROS. Antioxid Redox Signal. 2009;11:1289–99.
Gukovskaya AS, Vaquero E, Zaninovic V, et al. Neutrophils and NADPH oxidase mediate intrapancreatic trypsin activation in murine experimental acute pancreatitis. Gastroenterology. 2002;122:974–84.
Booth DM, Murphy JA, Mukherjee R, et al. Reactive oxygen species induced by bile acid induce apoptosis and protect against necrosis in pancreatic acinar cells. Gastroenterology. 2011;140:2116–25.
Schoenberg MH, Buchler M, Gaspar M, et al. Oxygen free radicals in acute pancreatitis of the rat. Gut. 1990;31:1138–43.
Abu-Zidan FM, Bonham MJ, Windsor JA. Severity of acute pancreatitis: a multivariate analysis of oxidative stress markers and modified Glasgow criteria. Br J Surg. 2000;87:1019–23.
Jones DP. Redefining oxidative stress. Antioxid Redox Signal. 2006;8:1865–79.
Alsfasser G, Gock M, Herzog L, et al. Glutathione depletion with L-buthionine-(S, R)-sulfoximine demonstrates deleterious effects in acute pancreatitis of the rat. Dig Dis Sci. 2002;47:1793–9.
Neuschwander-Tetri BA, Ferrell LD, Sukhabote RJ, Grendell JH. Glutathione monoethyl ester ameliorates caerulein-induced pancreatitis in the mouse. J Clin Invest. 1992;89:109–16.
Schulz HU, Niederau C, Klonowski-Stumpe H, Halangk W, Luthen R, Lippert H. Oxidative stress in acute pancreatitis. Hepatol Gastroenterol. 1999;46:2736–50.
Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP and ROS: a mitochondrial love-hate triangle. Am J Physiol-Cell Physiol. 2004;287:C817–33.
Pantano C, Reynaert NL, van der Vliet A, Janssen-Heininger YM. Redox-sensitive kinases of the nuclear factor-kappa β signalling pathway. Antioxid Redox Signal. 2006;8:1791–806.
Yang SR, Chida AS, Bauter MR, et al. Cigarette smoke induces proinflammatory cytokine release by activation of NF-kappa β and posttranslational modifications of histone deacetylase in macrophages. Am J Physiol Lung Cell Mol Physiol. 2006;291:L46–57.
Mukherjee R, Criddle DN, Gukovskaya A, Pandol S, Petersen OH, Sutton R. Mitochondrial injury in pancreatitis. Cell Calcium. 2008;44:14–23.
de Brito OM, Scorrano L. An intimate liaison: spatial organization of the endoplasmic reticulum-mitochondria relationship. EMBO J. 2010;29:2715–23.
Cardenas C, Miller RA, Smith I, et al. Essential regulation of cell bioenergetics by constitutive Ins P3 receptor Ca2+ transfer to mitochondria. Cell. 2010;142:270–83.
Pinton P, Giorgi C, Siviero R, ecchini E, Rizzuto R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene. 2008;27:6407–18.
Szmola R, Sahin-Toth M. Pancreatitis-associated chymotrypsinogen C (CTRC) mutant elicits endoplasmic reticulum stress in pancreatic acinar cells. Gut. 2009;59:365–72.
Lugea A, Tischler D, Nguyen J, et al. Adaptive unfolded protein response attenuates alcohol-induced pancreatic damage. Gastroenterology. 2011;140:987–97.
Malo A, Kruger B, Seyhun E, et al. Tauro ursodeoxycholic acid reduces endoplasmic reticulum stress, trypsin activation, and acinar cell apoptosis while increasing secretion in rat pancreatic acini. Am J Physiol Gastrointest Liver Physiol. 2010;299:G877–86.
Ye R, Mareninova OA, Barron E, et al. Grp78 heterozygosity regulates chaperone balance in exocrine pancreas with differential response to caerulein-induced acute pancreatitis. Am J Pathol. 2010;177:2827–36.
Gukovsky I, Gukovskaya AS, Blinman TA, Zaninovic V, Pandol SJ. Early NF-kappaβ activation is associated with hormone-induced pancreatitis. Am J Physiol. 1998;275:G1402–14.
Rakonczay Z Jr, Hegyi P, Takacs T, Saluja AK. The role of NF-kβ activation in the pathogenesis of acute pancreatitis. Gut. 2008;57:259–67.
Hietaranta AJ, Saluja AK, Bhagat L, Singh VP, Song AM, Steer ML. Relationship between NF-kappaβ and trypsinogen activation in rat pancreas after supra maximal caerulein stimulation. Biochem Biophys Res Commun. 2001;280:388–95.
Barnes PJ, Karin M. Nuclear factor-kappa β: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–71.
Satoh A, Gukovskaya AS, Nieto JMC, et al. PKC-delta and epsilon regulate NF-kappaβ activation induced by cholecystokinin and TNF alpha in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol. 2004;287:G582–91.
Hietaranta AJ, Singh VP, Bhagat L, et al. Water immersion stress prevents caerulein-induced pancreatic acinar cell NF-kappa β activation by attenuating caerulein-induced intracellular Ca2+ changes. J Biol Chem. 2001;276:18742–7.
Gukovskaya AS, Mouria M, Gukovsky I, et al. Ethanol metabolism and transcription factor activation in pancreatic acinar cells in rats. Gastroenterology. 2002;122:106–18.
Bhatia M, Brady M, Shokuhi S, Christmas S, Neoptolemos JP, Slavin J. Inflammatory mediators in acute pancreatitis. J Pathol. 2000;190:117–25.
Lin Y, Lin LJ, Jin Y, et al. Correlation between serum levels of high mobility group box-1 protein and pancreatitis: a meta-analysis. Biomed Res Int. 2015;2015:430185.
Wang W, Faubel S, Ljubanovic D, et al. Endotoxemic acute renal failure is attenuated in caspase-1-deficient mice. Am J Physiol Renal Physiol. 2005;288:F997–1004.
Zhang XH, Zhu RM, Xu WA, Wan HJ, Lu H. Therapeutic effects of caspase-1 inhibitors on acute lung injury in experimental severe acute pancreatitis. World J Gastroenterol. 2007;13:623–7.
Lampel M, Kern HF. Acute interstitial pancreatitis in the rat induced by excessive doses of a pancreatic secretagogue. Virchows Arch A Pathol Anat Histol. 1977;2:97–117.
Grady T, Liang P, Ernst SA, Logsdon CD. Chemokine gene expression in rat pancreatic acinar cells is an early event associated with acute pancreatitis. Gastroenterology. 1997;6:1966–75.
Demols A, Le Moine O, Desalle F, Quertinmont E, Van Laethem JL, Devière J. CD4+ T cells play an important role in acute experimental pancreatitis in mice. Gastroenterology. 2000;3:582–90.
Pietruczuk M, Dabrowska MI, Wereszczynska-Siemiatkowska U, Dabrowski A. Alteration of peripheral blood lymphocyte subsets in acute pancreatitis. World J Gastroenterol. 2006;12:5344–51.
Telek G, Ducroc R, Scoazec JY, Pasquier C, Feldmann G, Roze C. Differential up-regulation of cellular adhesion molecules at the sites of oxidative stress in experimental acute pancreatitis. J Surg Res. 2001;1:56–67.
Ostermann G, Weber KS, Zernecke A, Schroder A, Weber C. JAM-1 is a ligand of the β2-integrin LFA-1 involved in transendothelial migration of leukocytes. Nat Immunol. 2002;2:151–8.
Santoso S, Sachs UJ, Kroll H, et al. The junctional adhesion molecule 3 (JAM-3) on human platelets is a counterreceptor for the leukocyte integrin Mac-1. J Exp Med. 2002;5:679–91.
Powell JJ, Siriwardena AK, Fearon KC, Ross JA. Endothelial derived selectins in the development of organ dysfunction in acute pancreatitis. Crit Care Med. 2001;3:567–72.
Hartwig W, Werner J, Warshaw AL, et al. Membrane-bound ICAM-1 is up-regulated by trypsin and contributes to leukocyte migration in acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1194–9.
Zhang XP, Wang L, Zhou YF. The pathogenic mechanism of severe acute pancreatitis complicated with renal injury: a review of current knowledge. Dig Dis Sci. 2008;53:297–306.
Bhatia M, Neoptolemos JP, Slavin J. Inflammatory mediators as therapeutic targets in acute pancreatitis. Curr Opin Investig Drugs. 2001;2:496–501.
Dambrauskas Z, Giese N, Gulbinas A, et al. Different profiles of cytokine expression during mild and severe acute pancreatitis. World J Gastroenterol. 2010;16:1845–53.
Sathyanarayan G, Garg PK, Prasad HK, Tandon RK. Elevated level of interleukin-6 predicts organ failure and severe disease in patients with acute pancreatitis. J Gastroenterol Hepatol. 2007;22:550–4.
Zhang H, Neuhöfer P, Song L, et al. IL-6 trans-signaling promotes pancreatitis-associated lung injury and lethality. J Clin Invest. 2013;123:1019–31.
Bhatia M, Moochhala S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol. 2004;202:145–56.
Bhatia M, Wong FL, Cao Y, et al. Pathophysiology of acute pancreatitis. Pancreatology. 2005;5:132–44.
Norman JG, Fink GW, Franz MG. Acute pancreatitis induces intrapancreatic tumor necrosis factor gene expression. Arch Surg. 1995;130:966–70.
Fink GW, Norman JG. Specific changes in the pancreatic expression of the interleukin 1 family of genes during experimental acute pancreatitis. Cytokine. 1997;9:1023–7.
Suzuki S, Miyasaka K, Jimi A, Funakoshi A. Induction of acute pancreatitis by cerulein in human IL-6 gene transgenic mice. Pancreas. 2000;21:86–92.
Botoi G, Andercou A. Interleukin 17-prognostic marker of severe acute pancreatitis. Chirurgia (Bucur). 2009;104:431–8.
Perejaslov A, Chooklin S, Bihalskyy I. Implication of interleukin 18 and intercellular adhesion molecule (ICAM)-1 in acute pancreatitis. Hepatogastroenterology. 2008;55:1806–13.
Calandra T, Echtenacher B, Le Roy D, et al. Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nat Med. 2000;6:164–70.
Rau B, Steinbach G, Gansauge F, Mayer JM, Grunert A, Beger HG. The potential role of procalcitonin and interleukin 8 in the prediction of infected necrosis in acute pancreatitis. Gut. 1997;41:832–40.
Shokuhi S, Bhatia M, Christmas S, Sutton R, Neoptolemos JP, Slavin J. Levels of the chemokines growth-related oncogene alpha and epithelial neutrophil-activating protein 78 are raised in patients with severe acute pancreatitis. Br J Surg. 2002;89:566–72.
Rau B, Baumgart K, Kruger CM, Schilling M, Beger HG. CC-chemokine activation in acute pancreatitis: enhanced release of monocyte chemoattractant protein-1 in patients with local and systemic complications. Intensive Care Med. 2003;29:622–9.
Gerard C, Frossard JL, Bhatia M, Saluja A, Lu B, Steer ML. Targeted disruption of the beta-chemokine receptor CCR1 protects against pancreatitis-associated lung injury. J Clin Invest. 1997;100:2022–7.
Konturek SJ, Dembinski A, Konturek PJ, Warzecha Z, Jaworek J, Gustaw P. Role of platelet activating factor in pathogenesis of acute pancreatitis in rats. Gut. 1992;33:1268–74.
Bhatia M, Saluja AK, Hofbauer B, et al. Role of substance P and the neurokinin 1 receptor in acute pancreatitis and pancreatitis associated lung injury. Proc Natl Acad Sci U S A. 1998;95:4760–5.
Bhatia M, Wong FL, Fu D, Lau HY, Moochhala SM, Moore PK. Role of hydrogen sulfide in acute pancreatitis and associated lung injury. FASEB J. 2005;19:623–5.
Bhatia M, Saluja AK, Hofbauer B, Steer ML. Neutral endopeptidase (NEP) plays an anti-inflammatory role in acute pancreatitis and pancreatitis-associated lung injury. Pancreas. 1997;15:428.
Simovic MO, Bonham MJD, Abu-Zidan FM, Windsor JA. Anti-inflammatory cytokine response and clinical outcome in acute pancreatitis. Crit Care Med. 1999;27:2662–5.
Bhatia M, Singh VP, Frossard JL, Lee HS, Gerard C, Steer ML. Complement factor C5a exerts an anti-inflammatory effect in acute pancreatitis and associated lung injury. Am J Physiol Gastrointest Liver Physiol. 2001;280:G974–8.
Cuthbertson CM, Christophi C. Disturbances of the microcirculation in acute pancreatitis. Br J Surg. 2006;93:518–30.
Pitkaranta P, Kivisaari L, Nordling S, Nuutinen P, Schroder T. Vascular changes of pancreatic ducts and vessels in acute necrotizing, and in chronic pancreatitis in humans. Int J Pancreatol. 1991;8:13–22.
Foitzik T, Eibl G, Hotz HG, Faulhaber J, Kirchengast M, Buhr HJ. Endothelin receptor blockade in severe acute pancreatitis leads to systemic enhancement of microcirculation, stabilization of capillary permeability, and improved survival rates. Surgery. 2000;128:399–407.
Rudiger A, Singer M. Mechanisms of sepsis-induced cardiac dysfunction. Crit Care Med. 2007;35:1599–608.
Salomone T, Tosi P, Palareti G, et al. Coagulative disorders in human acute pancreatitis: role for the D-dimer. Pancreas. 2003;26:111–6.
Dixon B. The role of microvascular thrombosis in sepsis. Anaesth Intensive Care. 2004;32:619–29.
Radenkovic D, Bajec D, Ivancevic N, et al. D-dimer in acute pancreatitis: a new approach for an early assessment of organ failure. Pancreas. 2009;38:655–60.
Bleeker WK, Agterberg J, Rigter G, Hack CE, Gool JV. Protective effect of antithrombin III in acute experimental pancreatitis in rats. Dig Dis Sci. 1992;37:280–5.
Hackert T, Werner J, Gebhard MM, Klar E. Effects of heparin in experimental models of acute pancreatitis and post-ERCP pancreatitis. Surgery. 2004;135:131–8.
Farrant GJ, Abu-Zidan FM, Liu X, Delahunt B, Zwi LJ, Windsor JA. The impact of intestinal ischaemia-reperfusion on caerulein induced edematous experimental pancreatitis. Eur Surg Res. 2003;35:395–400.
Juvonen PO, Tenhunen JJ, Heino AA, et al. Splanchnic tissue perfusion in acute experimental pancreatitis. Scand J Gastroenterol. 1999;34:308–14.
Flint RS, Windsor JA. The role of the intestine in the pathophysiology and management of severe acute pancreatitis. HPB (Oxford). 2003;5:69–85.
Wu LM, Sankaran SJ, Plank LD, Windsor JA, Petrov MS. Meta-analysis of gut barrier dysfunction in patients with acute pancreatitis. Br J Surg. 2014;101:1644–56.
Ammori BJ. Role of the gut in the course of severe acute pancreatitis. Pancreas. 2003;26:122–9.
Fritz S, Hackert T, Hartwig W, et al. Bacterial translocation and infected pancreatic necrosis in acute necrotizing pancreatitis derives from small bowel rather than from colon. Am J Surg. 2010;200:111–7.
Garg PK, Khanna S, Bohidar NP, Kapil A, Tandon RK. Incidence, spectrum, and antibiotic sensitivity pattern of bacterial infections among patients with acute pancreatitis. J Gastroenterol Hepatol. 2001;16:1055–9.
Mentula P, Kylänpää ML, Kemppainen E, et al. Plasma anti-inflammatory cytokines and monocyte human leucocyte antigen-DR expression in patients with acute pancreatitis. Scand J Gastroenterol. 2004;39:178–87.
Beger HG, Bittner R, Block S, Buchler M. Bacterial contamination of pancreatic necrosis. A prospective clinical study. Gastroenterology. 1986;91:433–8.
Finfer SR, Vincent JL. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–51.
Mentula P, Kylänpää-Bäck ML, Kemppainen E, et al. Decreased HLA (human leucocyte antigen)-DR expression on peripheral blood monocytes predicts the development of organ failure in patients with acute pancreatitis. Clin Sci (Lond). 2003;105:409–17.
Lindström O, Kylänpää ML, Mentula P, et al. Upregulated but insufficient generation of activated protein C is associated with development of multiorgan failure in severe acute pancreatitis. Crit Care. 2006;10:R16.
Gotzinger P, Sautner T, Kriwanek S, et al. Surgical treatment for severe acute pancreatitis: extent and surgical control of necrosis determine outcome. World J Surg. 2002;26:474–8.
Bai Y, Gao J, Zou DW, Li ZS. Prophylactic antibiotics cannot reduce infected pancreatic necrosis and mortality in acute necrotizing pancreatitis: evidence from a meta-analysis of randomized controlled trials. Am J Gastroenterol. 2008;103:104–10.
Li JY, Yu T, Chen GC, et al. Enteral nutrition within 48 hours of admission improves clinical outcomes of acute pancreatitis by reducing complications: a meta-analysis. PLoS One. 2013;8, e64926.
Goldberg RF, Austen WG Jr, Zhang X, et al. Intestinal alkaline phosphatase is a gut mucosal defense factor maintained by enteral nutrition. Proc Natl Acad Sci U S A. 2008;105:3551–6.
Premkumar R, Phillips AR, Petrov MS, Windsor JA. The clinical relevance of obesity in acute pancreatitis: targeted systematic reviews. Pancreatology. 2015;15:25–33.
Yashima Y, Isayama H, Tsujino T, et al. A large volume of visceral adipose tissue leads to severe acute pancreatitis. J Gastroenterol. 2011;46:1213–8.
Noel P, Patel K, Durgampudi C, et al. Peripancreatic fat necrosis worsens acute pancreatitis independent of pancreatic necrosis via unsaturated fatty acids increased in human pancreatic necrosis collections. Gut. 2016;65:100–11.
Patel K, Trivedi RN, Durgampudi C, et al. Lipolysis of visceral adipocyte triglyceride by pancreatic lipases converts mild acute pancreatitis to severe pancreatitis independent of necrosis and inflammation. Am J Pathol. 2015;185:808–19.
Balog A, Gyulai Z, Boros LG, et al. Polymorphism of the TNF-alpha, HSP70-2, and CD14 genes increases susceptibility to severe acute pancreatitis. Pancreas. 2005;30:e46–50.
Papachristou GI, Sass DA, Avula H, et al. Is the monocyte chemotactic protein-1 -2518 G allele a risk factor for severe acute pancreatitis? Clin Gastroenterol Hepatol. 2005;3:475–81.
Johnson CD, Kingsnorth AN, Imrie CW, et al. Double blind, randomised, placebo controlled study of a platelet activating factor antagonist, lexipafant, in the treatment and prevention of organ failure in predicted severe acute pancreatitis. Gut. 2001;48:62–9.
Besselink MG, van Santvoort HC, Buskens E, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:651–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
PS, and PKG declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Singh, P., Garg, P.K. Pathophysiological mechanisms in acute pancreatitis: Current understanding. Indian J Gastroenterol 35, 153–166 (2016). https://doi.org/10.1007/s12664-016-0647-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12664-016-0647-y