Abstract
Recently it was reported that potato pulp, a side product of starch industry, is potentially applicable for the decontamination of phenol-polluted wastewater, due to its high peroxidase content. Regarding the toxicity and the persistence in the environment, the phenol is surpassed by its chlorinated derivatives, such as 2,4-dichlorophenol (2,4-DCP). In this study we demonstrated that potato pulp may be used for the decontamination of 2,4-DCP solutions in a peroxidase-catalyzed reaction. Due to its peroxidase activity, potato pulp displayed a very high potential for 2,4-DCP removal, with the reaction’s efficiency reaching 98% under optimal conditions. The peroxidase activity towards 2,4-DCP was maintained over a wide temperature and pH range, and characterized by relatively low H2O2 demand, with respect to other peroxidase-based systems of 2,4-DCP removal. The toxicity of the post-reaction solutions was compared to the toxicity of the unreacted 2,4-DCP solutions using phytotest and the MTT test. The results of both tests proved that the 2,4-DCP toxicity was effectively alleviated if the pollutant was depleted from solution in the course of the incubation with potato pulp and H2O2.
Similar content being viewed by others
Introduction
In an industrialized environment, a pressing matter of concern is the growing pollution. By the US EPA, phenol and some of its derivatives are classified as the priority pollutants, because of their toxicity in low doses, mutagenic, and teratogenic effects [1]. Among them, 2,4-dichlorophenol (2,4-DCP) is considered to be toxic and persistent in the environment, due to the presence of two halogenated atoms in the phenol ring [2]. Aside from being teratogenic and mutagenic, 2,4-DCP causes renal failure, pulmonary edema and anemia and is easily absorbed into human body [3]. Chlorophenols are abundant in the effluents and are released to the environment, for example, through the activity of pharmaceutical, metallurgic, and textile industry [4].
Conventional methods of removing 2,4-DCP from water and soil have been in use for many years and include chemical oxidation, solvent extraction, and liquid membrane permeation. However, they are usually very expensive and may lead to the formation of compounds more toxic than the initial pollutant [5]. Another popular method for toxic wastes removal is adsorption, which gives good results, but usually needs expensive activated carbon to work with high efficiency. Recently, agricultural wastes are used as a cheap adsorbent source, but the method has yet to gain popularity [6].
An interesting alternative to all those methods is a 2,4-DCP removal by hairy roots cultures. Such a culture is characterized by high genetic stability, high proliferation rate and an extensive contact surface with the toxic compound. However, maintaining hairy root cultures still generates costs [2, 3]. Another method utilizes plant peroxidases, which have an ability to oxidize phenolic compounds to fenoxyradicals, that later undergo spontaneous polymerization. Such polymers may be later removed from the medium by simple methods, like centrifugation [7]. The enzyme of choice is horseradish peroxidase (HRP). It is the best characterized peroxidase, and found many applications, including bioremediation, antibody conjugated enzyme production and immunoassays [8]. However, using purified enzyme preparations has its shortcomings—enzymes in this form are prone to inactivation and obtaining them is expensive. Thus, as an alternative to pure proteins, crude enzyme extracts or raw plant parts were considered as an enzyme source for remediation processes to lower the costs of removing pollutants [9, 10]. To improve the enzyme stability, the extracts may be immobilized in silicone, alginate, activated sludge or magnetic particles [6, 7, 11–13]. An alternative to HRP is soybean peroxidase—an enzyme with comparable thermal stability and susceptibility to inactivation [12]. Besides that, bitter gourd, radish and agricultural wastes were assessed as a source of peroxidases for remediation purposes [5, 9, 14].
The key to a widespread use of the alternative materials as enzyme sources is their availability and low cost. The above conditions are met by potato pulp—a waste product of starch industry, which production amounts to million tonnes per year in Europe. Our previous study showed, that potato pulp peroxidases were able to remove phenol from industrial and synthetic waste with high efficiency [15]. Since 2,4-DCP is characterized by higher persistency and toxicity, compared to phenol, we decided to test, whether potato pulp is a suitable material for its remediation.
In order to asses the usefulness of potato pulp for 2,4-DCP removal we optimized conditions of 2,4-DCP depletion to maximize the reaction efficiency and compared them with the published data for phenol. Furthermore, the post-reaction effluent was tested for toxicity to asses its impact on the environment.
Materials and Methods
Plant Material
Raw potato pulp was obtained from starch industry (PPZ Trzemeszno, Poland), portioned and frozen. The material was stored in −20 °C and thawed in room temperature directly before use.
The Conditions of 2,4-DCP Removal from Synthetic Effluent
2,4-DCP removal reactions were conducted in 15 ml polypropylene centrifuge tubes. The reaction mixture consisted of potato pulp, aqueous solution of 2,4-DCP and H2O2 in a total volume of 2.5 ml. The reaction tubes were incubated for 2 h with 200 rpm shaking, at 20 ± 2 °C. Standard reaction mixture for 2,4-DCP removal was composed of 100 mg of potato pulp, 1–3 mM 2,4-DCP and 2,59 mM H2O2. Samples were incubated for 2 h. Following the incubation, the residual 2,4-DCP was measured spectrophotometrically and the efficiency of its removal was calculated. As the reaction medium, tap water was used. 1, 2 and 3 mM 2,4-DCP solutions were prepared by dissolving 2,4-DCP powder in 0.5 ml of methanol and filling up with tap water to the desired volume. Stock solution of H2O2 was prepared from 30% H2O2 by diluting with deionized water.
The optimal conditions for the removal reactions were determined by testing the effect of selected experimental variables such as potato pulp sample weight, H2O2 and 2,4-DCP concentration, pH of the reaction mixture, shaking rate and incubation temperature on the efficiency of 2,4-DCP removal. Also, the stabilizing effect of PEG was assessed. The effect of 2,4-DCP concentration was assessed testing its removal efficiency at 1–3 mM concentration range, in the presence of 100 mg of potato pulp. The reaction was initiated by adding H2O2 to the final concentration of 2.59 mM. Simultaneously, the effect of polyethylene glycol (PEG) 3350 on the reaction efficiency was determined by adding PEG in the concentration of 100 and 200 mg/L to the reaction mixture. Following the incubation, the efficiency of 2,4-DCP removal was assessed.
Control Variants
Control reactions, where native potato pulp was replaced by autoclaved potato pulp, were carried on alongside test reactions, to take into account non-enzymatic 2,4-DCP degradation. The control reactions were conducted with 100 mg of autoclaved pulp, 2,4-DCP in the concentration range of 1–3 mM and 2.59 mM H2O2. Furthermore, reactions devoid of H2O2 were performed to exclude the possibility, that proteins other than peroxidases were involved in 2,4-DCP removal. The control reactions were incubated under the same conditions as test reactions namely 2 h in 20 ± 2 °C, with 200 rpm shaking.
H2O2 Determination
To determine the concentration of unreacted H2O2, its content was measured using 1 M potassium iodide. 500 µL of the post-reaction solution was added to 500 µL of tap water mixed with 1000 μL of 1 M KI. The absorbance of such mixture was measured at 390 nm and the concentration of H2O2 was compared with the calibration curve in the range of 0–1.75 mM H2O2.
The Effect of Potato Pulp Inoculum and H2O2 Concentration
The effect of potato pulp inoculum on 2,4-DCP removal was determined in the course of experiments where 100, 200, 300 and 400 mg of potato pulp were supplemented to the reaction mixtures composed of 1 mM 2,4-DCP solution and 2.59 mM H2O2. The effect of H2O2 concentration was determined, using 100 mg of potato pulp inoculum and supplementing them with 1 mM 2,4-DCP solution and H2O2 solutions from the concentration of 1.29–4.82 mM.
Effects of pH, Temperature and Shaking Rate on 2,4-DCP Removal
The assessment of pH effect on the removal efficiency was performed as described above, but the reaction medium was buffered to the pH values ranging from 2 to 10.
To assess the effect of temperature on 2,4-DCP removal, the samples were incubated in temperatures ranging from 10 to 60 °C for 2 h. In order to assess the effect of the shaking rate on the removal efficiency, the reaction tubes were incubated for 2 h under different shaking rates from 0 to 250 rpm. Then the residual 2,4-DCP was measured spectrophotometrically.
Phenols Determination
The residual 2,4-DCP after the reaction was measured according to the method of Kinsley and Nicell [16]. In this method, 5 μL of NH4OH, 5 μL of 2% 4-aminoantipyrine, and 10 µL of 8% potassium hexacyaniferrate are mixed with 1000 µL of post-reaction solution and incubated for 5 min. After that time, the absorbance of the mixture was measured at 510 nm and determined using the calibration curve in the range of 5–40 mg/L 2,4-DCP.
Garden Cress (Lepidium sativum) Toxicity Test
Garden cress seeds were preincubated in tap water for 1 h and then distributed onto Petri dishes padded with wet lignin. Ten seeds were placed into each plate. Preincubated seeds were treated with 3 mL of post reaction solutions and incubated for 48 h at room temperature. Simultaneously negative controls were performed by treating seeds with either 3 mL of unreacted 2,4-DCP solution or with 2,4-DCP solution incubated with potato pulp, but devoid of H2O2. The experiments included positive controls represented by seeds treated with tap water or water supplemented with 0.72 mM H2O2 (equivalent to the residual concentration of H2O2 after the reaction with 1 mM 2,4-DCP). After 48 h, the length of the seedlings’ roots was measured.
MTT Test
The viability of the cells and cytotoxicity effect were determined by the MTT assay with swine kidney (SK) cells, which is a colorimetric method based on the metabolic ability of the cells to reduce the yellow tetrazolium salt 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) to a blue crystalline formazan product. The number of viable cells after exposure is directly proportional to the amount of formazan produced, which can be quantified spectrophotometrically. Swine kidney (SK) cells were maintained in Dulbecco’s modified eagle’s medium (DMEM) supplemented with 5% foetal bovine serum, 1% antibiotic antimycotic solution (final concentrations: penicillin, 100 U/mL; streptomycin, 0.1 μg/mL) in a humidified atmosphere of 5% CO2 at 37 °C.
The samples were dissolved in a mixture of ethanol-dimethylsulfoxide-minimum essential medium with Earle’s salts (MEM) (1.7 + 0.3 + 98, v/v/v). Serial log2 dilutions of the sample were made. Dilutions were transferred to the assay plate of cells SK (number of cells 4.1 × 105 mL). All plates were incubated for 48 h at 37 °C in a humidified atmosphere with 5% CO2. A volume of 20 µL of the MTT stock solution was then added to each wells and plates were incubated for another 4 h. Supernatant was then removed using a multichannel micropipette and 100 µL DMSO were added to each well and measured spectrophotometrically with an ELISA-Reader. Microplate spectrophotometer (Elisa LEDETECT 96, Biogenet, Poland) and MikroWin 2000 (Biogenet, Poland) were used for quantitative evaluation of cytotoxicity. The absorbance was measured at 510 nm, the wavelength of maximum absorption of the formazan derivative. Since all the absorption values of the samples were below 50% of the division activity they were considered toxic. Therefore, on the basis of the levels of dilution the maximum acceptable toxic levels were determined, namely the smallest concentration of tested sample in (mg/mL) which had toxic effect on the cell (IC50).
On the basis of the subsequent steps of dilution cytotoxicity—IC50 value was determined, which is the sample concentration at which cell proliferation was inhibited by 50% compared to control cells. In the assessment of cytotoxicity there are used the values provided in the Table 1.
Statistics
Three independent replicates of each of the experiment were performed. Mean and the standard deviation were calculated. Statistical significances of the between differences in the results were assessed with one-way ANOVA (p = 0.05). All statistical analyses were performed with SigmaPlot 11.0 (Systat Software). The figures show representative results for each series of the experiments.
Results and Discussion
2,4-DCP Removal from Synthetic Wastewater
The ability of potato pulp peroxidases to remove 1–3 mM 2,4-DCP from the solution, was estimated by following the decrease in the 2,4-DCP concentration in the presence of potato pulp inoculum and H2O2. Furthermore, reactions with PEG 3350 supplemented to the final concentration of 100 or 200 mg/L were performed. To assess the specificity of the removal reaction, alongside the test samples, control reactions, devoid of H2O2, were conducted. Irrespective of the initial 2,4-DCP concentration tested, the pollutant was removed from the solution with very high efficiency, oscillating between 95 and 98% (Fig. 1). The efficiencies in 2,4-DCP removal, over the concentration range tested, were comparable, notwithstanding the statistical significance of differences between certain concentration variants (Fig. 1a, b). These results show that using potato pulp enables 2,4-DCP removal with the similar or higher efficiency with respect to other peroxidase-containing materials, Angelini et al. [3], showed that tobacco hairy roots extracts eliminated 2,4-DCP at 61.3 µM solution with the maximal removal efficiency reaching 92%. When garlic roots were tested for their ability to detoxify 2,4-DCP, the maximal removal efficiency of 97% was observed for 613 µM 2,4-DCP [1]. Transgenic alfalfa plants displayed the maximal efficiency (98.9%) of 2,4-DCP removal, over a 144 h-long treatment period, if 153 µM 2,4-DCP was supplemented to the reaction mixture [17]. The concentrations of 2,4-DCP, assessed by the aforementioned authors, were much lower than initial 2,4-DCP concentrations used in our study (1–3 mM). On the other hand, 100 mM 2,4-DCP was removed with the efficiency of 92% by shiitake mushroom [18] and when tobacco hairy roots were tested, efficient 2,4-DCP degradation was possible even for 1000 mM pollutant solutions [2].
Removal efficiencies at different 2,4-DCP concentrations. The reaction was performed in a medium composed of 100 mg of potato pulp, 2,4-DCP solution and 2.59 mM H2O2. The control mixtures (pulp + 1, 2 or 3 mM 2,4-DCP) were devoid of H2O2. The reactions were conducted in the presence (grey bars) or absence (black bars) of PEG 3350 in the concentration 100 mg/L (a) or 200 mg/L (b). Different letters denote significant differences at p ≤ 0.05
The prerequisite for the efficient 2,4-DCP removal was supplementing the reaction mixture with both potato pulp and H2O2. Contrastingly, the H2O2-independent removal of 2,4-DCP was significantly lower for all assays (Fig. 1). Therefore we conclude, that the 2,4-DCP removal was strongly dependent on peroxidase activity. To provide further evidence for the enzymatic mechanism of 2,4-DCP removal, we performed reactions with autoclaved potato pulp added instead of the native one (Fig. 2). The assays with the autoclaved pulp were performed in the same manner as those with native potato pulp, i.e. the H2O2-supplemented test samples were accompanied by H2O2-unsupplemented control variants. Under these conditions, 2,4-DCP removal did not exceed 55%, when the reaction mixtures were supplemented with H2O2. Concurrently, up to 30% 2,4-DCP was removed in H2O2-unsupplemented controls. No significant differences were observed between samples initially supplemented with different 2,4-DCP concentrations. The partial 2,4-DCP removal in H2O2-unsupplemented mixtures might be due to the adsorption on the potato pulp surface. A non-enzymatic oxidation by H2O2 might also occur in assay mixtures supplemented with this oxidant, since adding H2O2 to the reaction medium increased 2,4-DCP removal efficiency in the presence of autoclaved pulp (but not in a statistically significant way) (Fig. 2). These findings are corresponding with data reported in our previous study, where it was shown that the removal of phenol by potato pulp did not exceed 55% in H2O2-unsupplemented reaction mixtures, but amounted to 99%, given that both potato pulp and H2O2 were present in the reaction medium [15]. On the other hand, Wang et al. [1] observed, that the H2O2-independent 2,4-DCP removal by raw or autoclaved garlic roots was at the level of 18–27%. Similar results were obtained for tobacco hairy roots, where H2O2-independent 2,4-DCP removal reached 18%, whereas autoclaved roots incubated with 2,4-DCP and H2O2 displayed 23% pollutant removal [2].
It was shown, that adding PEG had beneficial effect on removal of phenol and phenol derivatives by extracted peroxidases [9]. Also, for hairy root cultures, Angelini et al. [3] reported, that adding PEG 3350 to the reaction solution improved the efficiency of pollutant removal. However, similarly to González et al. [19], we did not observe any statistically significant differences, in the removal efficiency, between the reaction variant with and without PEG supplementation (Fig. 1a, b). The previous experiments with phenol also did not show any relation between phenol removal and the presence of PEG in the reaction mixture [15]. PEG acts through preventing product-dependent denaturation of peroxidases [20]. Similar, protection and increase of efficacy was obtained by immobilizing purified peroxidases on silica or magnetic particles [11, 21]. Immobilization itself is known to stabilize enzymes [22], thus it is possible, that similar protection to potato pulp peroxidases is provided by cell wall material which they are immobilized on.
Residual H2O2
To assess the possibility, that the post-reaction effluent might contain toxic concentrations of unreacted hydrogen peroxide, we determined the residual concentration of H2O2, after the reactions were completed. The residual H2O2 concentration was dependent on the initial 2,4-DCP concentration in the assay media. We observed, that the concentration of H2O2 in post-reaction medium amounted to 0.718 ± 0.009 mM if 2,4-DCP was depleted from 1 mM solution, whereas mixtures initially supplemented with 2 or 3 mM 2,4-DCP retained much lower H2O2 concentrations, respectively 0.235 ± 0.027 and 0.026 ± 0.001 mM. For comparison, if 1 mM phenol was removed by potato pulp in a peroxidase-catalyzed reaction, the concentration of residual H2O2 amounted to 0.546 ± 0.09 mM [15]. The reported data stress the importance of the residual H2O2 in post reaction solutions, when the safety of peroxidase-treated wastewaters is considered.
Effect of the Potato Pulp Weight Inoculum and Hydrogen Peroxide Concentration
Effect of potato pulp sample weight on the efficiency of the 2,4-DCP removal was tested by supplementing the potato pulp in the range of 100–400 mg per 2.5 mL of reaction solution. There were no statistically significant differences between the variants tested, with the removal efficiency from nearly 95 to 98%, hence 100 mg of potato pulp was chosen for further experiments (Fig. 3). The potato pulp inoculum necessary for the removal of 2,4-DCP with high efficiency was lower than the one required for phenol depletion. As reported in our previous work, the maximal efficiency of phenol removal required three times higher potato pulp inoculum [15], compared to 2,4-DCP (Fig. 3). In adsorption studies on 2,4-DCP removal, where pomegranate (Punica granatum) peels were used, 100 mg of adsorbent was used with 10 mL of 2,4-DCP solution. However, the reaction was conducted for 10 h [6]. On the other hand, when garlic roots were used as a peroxidase source, 400 mg of fresh plant material was incubated in 10 mL of reaction solution to assure the efficient depletion of 2,4-DCP from solution [1].
The effect of hydrogen peroxide concentration was tested by estimating the efficiency of the 2,4-DCP depletion in the presence of the series of H2O2 concentrations. The reaction media were supplemented with 100 mg of potato pulp. We observed, that 1.94 mM H2O2 was the lowest concentration which enabled 2,4-DCP removal with maximal efficiency. At higher H2O2 concentrations the efficiency of the process remained unchanged (Fig. 4). Nevertheless, the concentration of 2.59 mM was chosen for further experiments, as the one corresponding better with the previous experiments with phenol [15]. The reaction mixtures completely devoid of H2O2 exhibited much lower 2,4-DCP removal (33.5%), whereas the optimal concentration of hydrogen peroxide ensured removal efficiency at the level of nearly 98% (Fig. 4). Our experimental set was characterized by a much lower H2O2 demand compared to the one of Wang and co-workers [1], who found 10 mM H2O2 optimal for the removal of 2,4-DCP from wastewater by garlic roots [1]. Similar, removing 2,4-DCP with peroxidase-producing tobacco hairy roots required 10 mM H2O2 for optimal efficiency [2].
Effect of pH on 2,4-DCP Removal
The effect of the reaction mixture pH was tested in the range from 2 to 10 in the presence of 1, 2 or 3 mM initial 2,4-DCP concentration (Fig. 5). Under all 2,4-DCP concentrations used, the reaction carried on at pH 2 exhibited the lowest removal efficiency, amounting to 48% (Fig. 5a, b, c). Reactions performed in pH 4–10 did not display significant differences between the experimental variants tested, showing the 2,4-DCP removal efficiency at the level of 97–98%. Alongside the test reactions, the controls devoid of H2O2 were performed, with the highest removal efficiency amounting to 55%, but mostly not exceeding 40% (Fig. 5a, b, c). The optimal pH for 2,4-DCP removal due to peroxidase activity associated with garlic roots reported by Wang et al. [1] was 7, whereas Bhatnagar and Minocha [6] showed that 2,4-DCP depletion in an adsorption-based process required pH ranging from 5.5 to 6.5. Potato pulp peroxidases retained their activity against 2,4-DCP over much wider range of pH, when compared to the aforementioned data, with no visible activity losses even in pH 10 (Fig. 5). On the other hand, the efficiency of phenol removal, was reduced to 60%, at this pH value [15].
The effect of different pH on 2,4-DCP removal. The reaction mixtures were composed of 100 mg of potato pulp, 2.59 mM H2O2 and 1 mM 2,4-DCP solution (a), 2 mM 2,4-DCP solution (b) or 3 mM 2,4-DCP solution (c). Besides the reactions (black dots), control variants devoid of H2O2 (white dots) were performed. Different letters denote significant differences at p ≤ 0.05
Effect of Temperature on 2,4-DCP Removal
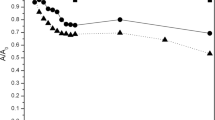
The effect of the temperature was tested in the range of 10 to 60 °C using 1, 2 or 3 mM 2,4-DCP reaction solution as a reaction medium. We observed, that the removal efficiency did not drop below 95% irrespectively of the incubation temperature used. Even at the highest and lowest temperatures applied, a very high rate of 2,4-DCP was maintained (Fig. 6). When the reaction solution supplemented with 1 mM 2,4-DCP was tested, no significant differences were detected throughout the entire temperature range (Fig. 6a). In the case of 2 mM 2,4-DCP, the highest, over 97% removal efficiency was observed at 50 °C (Fig. 6b), and for 3 mM 2,4-DCP the highest percentage of removal reaction was observed at 60 °C, with the reaction efficiency at the level of over 96% (Fig. 6c). However, none of the differences were significant, showing that potato pulp peroxidases display high thermal stability and maintained their activity towards 2,4-DCP over a wide range of temperatures. Those results correspond with previous data concerning phenol removal, when the efficient degradation of this pollutant by potato pulp peroxidases, over wide temperature range was demonstrated [15]. The efficiency of 2,4-DCP removal from wastewaters has not been assessed against temperature range yet. Angelini et al. [3], Bhatnagar and Minocha [6] and Wang et al. [1] performed their reactions at 25 °C. Interestingly, potato pulp was more resistant to thermal inactivation of the peroxidase activity than commercial peroxidases immobilized on silicone, that lost 90% of their activity in 60 °C. Soluble form of the same peroxidase retained 40% of its activity in 60 °C [7].
Effect of Shaking Rate
The intensity of shaking the wastewater during treatment with potato pulp may affect the energy demand, and consequently the operating cost of the future large-scale applications of this industrial waste for the remediation purposes. The effect of shaking rate on the 2,4-DCP removal efficiency was tested against controls, which were mixed thoroughly immediately after the reaction mixtures were composed, and then left on the bench. Simultaneously, the treated samples were subjected to different shaking intensities. Two h long continuous shaking of the rate ranging from 50 to 200 rpm was the variable (Fig. 7). Irrespectively of the initial 2,4-DCP concentration, the unshaken control displayed the lowest 2,4-DCP removal efficiency compared to shaken samples. Nevertheless, the unshaken controls still displayed a very high degree of 2,4-DCP depletion ranging from almost 91–93.5% (Fig. 7a, b, c). The differences in the efficiency of the pollutant removal from potato pulp-treated wastewater, between the shaken and unshaken samples, were more distinguished when treating phenol solution. The unshaken control in phenol-removal experiments displayed the efficiency at the level of 70% [15]. Shaking the samples, initially supplemented with 2 or 3 mM 2,4-DCP resulted in the same removal efficiency over the entire range of shaking rates applied, without any statistically significant differences between the samples that received shaking of different intensity (Fig. 7b, c). The reaction mixture supplemented with 1 mM 2,4-DCP, required a shaking rate of at least 100 rpm to show maximal removal efficiency (Fig. 7a). Nevertheless, the differences between the samples that received shaking of different intensities were negligible and under most shaking rates, the 2,4-DCP removal efficiency surpassed 98% (Fig. 7). To the best of our knowledge, range of shaking rates was not usually tested, when exploring the peroxidase-mediated 2,4-DCP removal, with 100 rpm being most commonly applied [1, 3].
Lepidium sativum Toxicity Test
In order to determine, whether our method alleviated the toxic effect of 2,4-DCP on plants, we performed a toxicity test on garden cress seeds. The root length of seedlings developed from seeds treated with the post reaction solutions initially supplemented with 1–3 mM 2,4-DCP and incubated with both potato pulp inoculum and 0.718 mMH2O2 (the concentration of residual H2O2) was compared to those treated with unreacted 2,4-DCP solutions or 2,4-DCP solutions subjected to incubation with potato pulp but unsupplemented with H2O2. Plants germinated in the presence of tap water were considered as positive controls. The assay was complemented with the bulk of seedlings incubated with the aqueous solution of H2O2 corresponding to its residual concentration after the 2,4-DCP removal reaction (Fig. 8). After 48 h of incubation, seedlings treated either with unreacted 2,4-DCP solutions or reaction mixtures devoid of H2O2 did not germinate at all. Contrastingly the seedlings treated with post-reaction solutions representing all initial 2,4-DCP concentrations displayed roots’ length at the level of positive control (Fig. 8). Hence, we concluded, that application of our method of 2,4-DCP removal from aqueous solution effectively alleviated toxic effects of 2,4-DCP on plants.
Toxicity test on Lepidium sativum. After 48 h of incubation, mean length of seedlings’ root is shown. The seedlings were treated with 0.718 mM H2O2, 1–3 mM 2,4-DCP post-reaction solutions, 1–3 mM 2,4-DCP solutions with pulp, devoid of H2O2 (2,4-DCP + pulp) and unreacted 1–3 mM 2,4-DCP solutions. Different letters denote significant differences at p ≤ 0.05
Similar to our results, Wang et al. [1] reported that toxicity of 2,4-DCP solution was reduced due to the treatment with peroxidase-containing plant tissue. Using garlic roots as a source of peroxidase, the authors demonstrated that, that the inhibitory effect of 2,4-DCP on Lactuca sativa seed germination and seedling growth was decreased by removing the pollutant during a peroxidase-catalyzed reaction [1]. The toxicity test on L. sativum highlighted more toxic effect of 2,4-DCP on plants, compared to phenol [15]. In contrast to 2,4-DCP, the equimolar phenol solutions did not prevent seed germination. Nevertheless, strong inhibition of the root growth, under treatments with increasing phenol concentration, was observed [15].
We did not observe any statistically significant differences between control seedlings and the ones treated with H2O2 solution (Fig. 8). This finding suggests, that residual H2O2 in the post reaction mixture did not have toxic effect on L. sativum seedlings. Our results corresponded to those reported by Angelini et al. [3], who showed that H2O2 in the concentration of 0.5 mM did not inhibit the growth of L. sativa seedlings.
MTT Toxicity Test
Chen et al. demonstrated high cytotoxicity of chlorinated phenols using MTT test. They reported, that 2,4-DCP at 0.3 mM concentration decreased cell viability to about 25% [23]. In this study 2 or 3 mM2,4-DCP displayed the highest cytotoxicity with the IC50 of 31.25 µL/mL. The same toxicity level was maintained if cells were treated with 2 and 3 mM 2,4-DCP preincubated with potato pulp, under the absence of H2O2. The reduction in the cytotoxicity of 2,4-DCP was observed if the solutions were pretreated with potato pulp in the presence of H2O2. IC50 for the post-reaction mixture initially supplemented with 3 mM 2,4-DCP was determined to be 62.5 µL/mL and the one supplemented with 2 mM 2,4-DCP did not show the cytotoxicity at the detectable level (Table 2). Untreated 1 mM 2,4-DCP solution displayed lower toxicity than 2 or 3 mM 2,4-DCP, with IC50 62.5 µl/mL. This value was not changed after 1 mM 2,4-DCP was treated with pulp and H2O2, but the solution did not display any detectable toxicity if incubated with potato pulp devoid of H2O2 (Table 2). The results of the MTT test were mostly consistent with those obtained with L. sativum toxicity test. Both tests show the reduction in the 2,4-DCP toxicity if the pollutant was depleted from solution in the course of the incubation with potato pulp and H2O2 (Fig. 8; Table 2).
Conclusion
This study has shown the great efficiency of potato pulp to remove of 2,4-DCP. The 2,4-DCP removal efficiency ranged from 95 to 98% when 1–3 mM 2,4-DCP was used. The removal process was strongly dependent on the presence of H2O2 in the reaction medium, which suggests that peroxidase-based mechanism is implicated in depletion of the pollutant from treated water. A bioremediation system should not only be cheap and scalable, but also versatile. Despite the enzymatic mechanism involved, the removal reaction was highly efficient under wide range of temperatures and pH values. The detoxifying effect of 2,4-DCP oxidation was assessed by toxicity test. It was demonstrated that 2,4-DCP solutions treated with potato pulp showed significant reduction in toxicity with respect to untreated 2,4-DCP solutions. So far, using potato pulp as a peroxidase source has yielded high pollutant removal efficiencies for both phenol [15] and 2,4-DCP. Furthermore, using this peroxidase-containing material provides a convenient method for industrial dyes removal (data not shown), which makes potato pulp peroxidases a good alternative to methods exploited nowadays.
References
Wang, Y., Zhang, J.X., Ren, H.J., Wang, Y., Pan, H.Y., Zhang, L.Y.: Phytoremediation potentiality of garlic roots for 2,4-dichlorophenol removal from aqueous solutions. Appl. Microbiol. Biotechnol. 99, 3629–3637 (2015)
Talano, M.A., Frontera, S., González, P., Medina, M.I., Agostini, E.: Removal of 2,4-dichlorophenol from aqueous solutions using tobacco hairy root cultures. J. Hazard. Mater. 176, 784–791 (2010)
Angelini, V.A., Agostini, E., Medina, M.I., González, P.S.: Use of hairy roots extracts for 2,4-DCP removal and toxicity evaluation by Lactuca sativa test. Environ. Sci. Pollut. Res. 21, 2531–2539 (2014)
Michałowicz, J., Bukowska, B., Duda, W.: The differences in phenolics content in rivers exposed and non-exposed to anthropogenic contamination. Chemosphere. 71, 735–741 (2008)
Akhtar, M., Iqbal, M.I., Hasany, S., S.M: Sorption potential of rice husk for the removal of 2,4-dichlorophenol from aqueous solutions: kinetic and thermodynamic investigations. J. Hazard. Mater. 128, 44–52 (2006)
Bhatnagar, A., Minocha, A.K.: Adsorptive removal of 2,4-dichlorophenol from water utilizing Punica granatum peel waste and stabilization with cement. J. Hazard. Mater. 168, 1111–1117 (2009)
Sahare, P., Ayala, M., Vazquez-Duhalt, R., Agrawal, V.: Immobilization of peroxidase enzyme onto the porous silicon structure for enhancing its activity and stability. Nanoscale Res. Lett. 9, 409–417 (2014)
Regalado, C., García-Almendárez, B.E., Duarte-Vázquez, M.A.: Biotechnological applications of peroxidases. Phytochem. Rev. 3, 243–256 (2004)
Diao, M., Ouédraogo, N., Baba-Moussa, L., Savadogo, P.W., N’Guessan, A.G., Bassolé, I.H.N., Dicko, M.H.: Biodepollution of wastewater containing phenolic compounds from leather industry by plant peroxidases. Biodegradation. 22, 389–396 (2011)
Sharma, S., Mukhopadhyay, M., Murthy, Z.V.P: Investigation of photo-assisted and crude peroxidase mediated transformations of chlorinated phenols (CPs) from spiked and industrial wastewaters: identification of reaction products. Water Sci. Technol. 72, 746–753 (2015)
Chang, Q., Tang, H.: Immobilization of horseradish peroxidase on NH2-modified magnetic Fe3O4/SiO2 particles and its application in removal of 2,4-dichlorophenol. Molecules. 19, 15768–15782 (2014)
Prokopijevic, M., Prodanovic, O., Spasojevic, D., Stojanovic, Z., Radotic, K., Prodanovic, R.: Soybean hull peroxidase immobilization on macroporous glycidyl methacrylates with different surface characteristics. Bioproc. Biosys. Eng. 37, 799–804 (2014)
Singh, S., Melo, J.S., Eapen, S., D’Souza, S.F.: Potential of vetiver (Vetiveria zizanoides L. Nash) for phytoremediation of phenol. Ecotox. Environ. Saf. 71, 671–676 (2008)
El Agha, A., Makris, D.P., Kefalas, P.: Peroxidase-active cell free extract from onion solid wastes: biocatalytic properties and putative pathway of ferulic acid oxidation. J. Biosci. Bioeng. 106, 279–285 (2008)
Kurnik, K., Treder, K., Skorupa-Kłaput, M., Tretyn, A., Tyburski, J.: Removal of phenol from synthetic and industrial wastewater by potato pulp peroxidases. Water Air Soil Pollut. 226, 254–272 (2015)
Kinsley, C., Nicell, J.A.: Treatment of aqueous phenol with soybean peroxidase in the presence of polyethylene glycol. Bioresour. Technol. 73, 139–146 (2000)
Wang, Y., Ren, H., Pan, H., Liu, J., Zhang, L.: Enhanced tolerance and remediation to mixed contaminates of PCBs and 2,4-DCP by transgenic alfalfa plants expressing the 2,3-dihydroxybiphenyl-1,2-dioxygenase. J. Hazard. Mater. 286, 269–275 (2015)
Tsujiyama, S., Muraoka, T., Takada, N.: Biodegradation of 2,4-dichlorophenol by shiitake mushroom (Lentinula edodes) using vanillin as an activator. Biotechnol. Lett. 35, 1079–1083 (2013)
González, P.S., Agostini, E., Milrad, S.R.: Comparison of the removal of 2,4-dichlorophenol and phenol from polluted water, by peroxidases from tomato hairy roots, and protective effect of polyethylene glycol. Chemosphere. 70, 982–989 (2008)
Quintanilla-Guerrero, F. ,Duarte-Vázquez, M.A., García-Almendarez, B.E., Tinoco, R., Vazquez-Duhalt, R., Regalado, C.: Polyethylene glycol improves phenol removal by immobilized turnip peroxidase. Bioresour. Technol. 99, 8605–8611 (2008)
Yang, J., Huang, Y., Yang, Y., Yuan, H., Liu, X.: Cagelike mesoporous silica encapsulated with microcapsules for immobilized laccase and 2, 4-DCP degradation. J. Environ. Sci. 38, 52–62 (2015)
Alemzadeh, I., Nejati, S.: Phenols removal by immobilized horseradish peroxidase. J. Hazard. Mater. 166, 1082–1086 (2009)
Chen, J., Jiang, J., Zhang, F., Yu, H., Zhang, J.: Cytotoxic effects of environmentally relevant chlorophenols on L929 cells and their mechanisms. Cell Biol. Toxicol. 20, 183–196 (2004)
Acknowledgements
Authors wish to thank MSc Ewelina Soszczyńska for helping with cytotoxicity evaluation. This study was financially supported by a grant from the National Science Centre, Poland (Grant No. 2014/13/B/NZ9/03790).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kurnik, K., Treder, K., Twarużek, M. et al. Potato Pulp as the Peroxidase Source for 2,4-Dichlorophenol Removal. Waste Biomass Valor 9, 1061–1071 (2018). https://doi.org/10.1007/s12649-017-9863-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-017-9863-7