Abstract
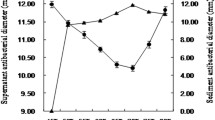
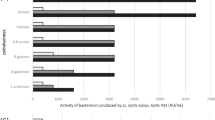
The lactic acid bacteria (LAB) microbiota of Saudi chicken ceca was determined. From 60 samples, 204 isolates of lactic acid bacteria were obtained. Three isolates produced antimicrobial activities against Campylobacter jejuni, Listeria monocytogenes, and Bacillus subtilis. The isolate DN317, which had the highest activity against Campylobacter jejuni ATCC 33560, was identified as Lactobacillus curvatus (GenBank accession numbers: KX353849 and KX353850). Full inhibitory activity was observed after a 2-h incubation with the supernatant at pH values between 4 and 8. Only 16% of the activity was conserved after a treatment at 121 °C for 15 min. The use of proteinase K, pepsin, chymotrypsin, trypsin, papain, and lysozyme drastically reduced the antimicrobial activity. However, lipase, catalase, and lysozyme had no effect on this activity. The active peptide produced by Lactobacillus curvatus DN317 was purified by precipitation with an 80% saturated ammonium sulfate solution, and two steps of reversed phase HPLC on a C18 column. The molecular weight of this peptide was 4448 Da as determined by MALDI-ToF. N-terminal sequence analysis using Edman degradation revealed 47 amino acid residues (UniProt Knowledgebase accession number C0HK82) revealing homology with the amino acid sequences of sakacin P and curvaticin L442. The antimicrobial activity of the bacteriocin, namely curvaticin DN317, was found to be bacteriostatic against Campylobacter jejuni ATCC 33560. The use of microbial antagonism by LAB is one of the best ways to control microorganisms safely in foods. This result constitutes a reasonable advance in the antimicrobial field because of its potential applications in food technology.



Similar content being viewed by others
References
Scallan E, Hoekstra RM, Mahon BE, Jones TF, Griffin PM (2015) An assessment of the human health impact of seven leading foodborne pathogens in the United States using disability adjusted life years. Epidemiol Infect 143:2795–2804
Lake RJ, Cressey PJ, Campbell DM, Oakley E (2010) Risk ranking for foodborne microbial hazards in New Zealand: burden of disease estimates. Risk Anal 30:743–752
Mangen MJJ, Bouwknegt M, Friesema IHM, Haagsma JA, Kortbeek LM, Tariq L (2015) Cost-of-illness and disease burden of food-related pathogens in the Netherlands. Int J Food Microbiol 196:84–93
Gibney KB, O’Toole J, Sinclair M, Leder K (2014) Disease burden of selected gastrointestinal pathogens in Australia. Int J Infect Dis 28:176–185
Skarp CPA, Hänninen ML, Rautelin HIK (2016) Campylobacteriosis: the role of poultry-meat. Clin Microbiol Infect 22:103–109
Apajalahti J, Kettunen A, Graham H (2004) Characteristics of the gastrointestinal microbial communities, with special reference to the chicken. World’s Poult Sci J 60:223–232
Bjerrum L, Engberg RM, Leser TD, Jensen BB, Finster K, Pedersen K (2006) Microbial community composition of the ileum and cecum of broiler chickens as revealed by molecular and culture based techniques. Poult Sci 85:1151–1164
Shaughnessy RG, Meade KG, McGivney BA, Allan B, O’Farrelly C (2011) Global gene expression analysis of chicken caecal response to Campylobacter jejuni. Vet Immunol Immunopathol 142:64–71
Khin KZ, Saelao P, Halstead MM, Chanthavixay G, Chang HC (2015) Salmonella enterica Serovars Enteritidis infection alters the indigenous microbiota diversity in young layer chicks. Front Vet Sci 2:1–16
Anastasiadou S, Papagianni M, Filiousis G, Ambrosiadis I, Koidis P (2008) Growth and metabolism of a meat isolated strain of Pediococcus pentosaceus in submerged fermentation: purification, characterization and properties of the produced pediocin SM-1. Enzym Microb Technol 43:448–454
Papagianni M, Anastasiadou S (2009) Pediocins: the bacteriocins of pediococci. Sources, production, properties and applications. Microb Cell Factories 8:3
Klaenhammer TR (1993) Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol Rev 12:39–86
Atanassova V, Meindl A, Ring C (2001) Prevalence of Staphylococcus aureus and staphylococcal enterotoxins in raw pork and uncooked smoked ham: a comparison of classical culturing detection and RFLP-PCR. Int J Syst Evol Microbiol 68:105–113
Cotter PD, Hill C, Ross RP (2005) Bacteriocins: developing innate immunity for food. Nat Rev Microbiol 3:777–788
Balciunas EM, Castillo Martinez FA, Todorov SD, Franco BDGM, Converti A (2013) Novel biotechnological applications of bacteriocins: a review. Food Control 32:134–142
Heng NCK, Wescombe PA, Burton JP, Jack RW, Tagg JR (2007) Bacteriocins: ecology and. Evolution:45–92
Abriouel H, Valdivia E, Martinez-Bueno M, Maqueda M, Galvez A (2003) A simple method for semi-preparative-scale production and recovery of enterocin AS-48 derived from Enterococcus faecalis subsp. liquefaciens A-48-32. J Microb Methods 55:599–605
Chaillou S, Champomier-Vergès MC, Cornet M, Crutz-Le Coq A, Dudez AM (2005) The complete genome sequence of the meat-borne lactic acid bacterium Lactobacillus sakei 23 K. Nat Biotechnol 23:1527–1533
Castellano P, Gonzalez C, Carduza F, Vignolo G (2010) Protective action of Lactobacillus curvatus CRL705 on vacuum-packaged raw beef. Effect on sensory and structural characteristics. Meat Sci 85:394–401
Castellano P, Belfiore C, Fadda S, Vignolo G (2008) A review of bacteriocinogenic lactic acid bacteria used as bioprotective cultures in fresh meat produced in Argentina. Meat Sc 79:483–499
Ercolini D, Russo F, Torrieri E, Masi P, Villani F (2006) Changes in the spoilage-related microbiota of beef during refrigerated storage under different packaging conditions. Appl Environ Microbiol 72:4663–4671
Lyhs U, Korkeala H, Bjorkroth J (2002) Identification of lactic acid bacteria from spoiled, vacuum-packaged ‘gravad’ rainbow trout using ribotyping. Int J Food Microbiol 72:147–153
Berthier F, Ehrlich SD (1999) Genetic diversity within Lactobacillus sakei and Lactobacillus curvatus and design of PCR primers for its detection using randomly amplified polymorphic DNA. Int J Syst Bacteriol 49:997–1007
Fadda S, Lopez C, Vignolo G (2010) Role of lactic acid bacteria during meat conditioning and fermentation: peptides generated as sensorial and hygienic biomarkers. Meat Sci 86:66–79
Stern NJ, Svetoch EA, Eruslanov BV, Kovalev YN, Volodina LI, Perelygin VV (2005) Paenibacillus polymyxa purified bacteriocin to control Campylobacter jejuni in chickens. J Food Prot 68:1450–1453
Messaoudi S, Kergourlay G, Dalgalarrondo M, Ferchichi M, Prévost H, Manai M (2012) Purification and characterization of a new bacteriocin active against Campylobacter produced by Lactobacillus salivarius SMXD51. Food Microbiol 32:129–134
Messaoudi S, Kergourlay G, Rossero A, Ferchichi M, Prévost H, Drider D (2011) Identification of lactobacilli residing in chicken ceca with antagonism against Campylobacter. Int Microbiol 14:103–110
De Man JCA, Rogosa M, Sharpe ME (1964) A medium for the cultivation of lactobacilli. J Appl Bacteriol 23:130–136
Kabadjova P, Dousset X, Le Cam V, Prevost H (2002) Differentiation of closely related Carnobacterium food isolates based on 16S-23S ribosomal DNA intergenic spacer region polymorphism. Appl Environ Microbiol 68:5358–5366
Dubernet S, Desmasures N, Guéguen M (2002) A PCR-based method for identification of lactobacilli at the genus level. FEMS Microbiol Letters 21:271–275
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173:697–703
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Todorov SD, Dicks LM (2005) Optimization of bacteriocin ST311LD production by Enterococcus faecium ST 311LD, isolated from spoiled black olives. J Microbiol 43:370–374
Mitéva V, Ivanova I, Budakov I, Pantev A, Stefanoa T (1998) Detection and characterization of a novel antimicrobial substance produced by a Lactobacillus delbrueckii strain 1043. J Appl Microbiol 85:603–614
Schillinger U, Lücke FK (1989) Antibacterial activity of Lactobacillus sake isolated from meat. Appl Environ Microbiol 55:1901–1906
Althschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Gong J, Forster RJ, Yu H, Chambers JR, Wheatcroft R, Sabour PM, Chen S (2002) Molecular analysis of bacterial populations in the ileum of broiler chickens and comparison with bacteria in the cecum. FEMS Microbiol Ecol 41:171–179
Lu X, Rasco BA, Jabal JM, Aston DE, Lin M, Konkel ME (2011) Investigating antibacterial mechanisms of garlic (Allium sativum) concentrate and garlic-derived organosulfur compounds on Campylobacter jejuni using FT-IR spectroscopy, Raman spectroscopy and electron microscope. Appl Environ Microbiol 72:799–805
Dumonceaux TJ, Hill JE, Hemmingsen SM, Van Kessel AG (2006) Characterization of intestinal microbiota and response to dietary virginiamycin supplementation in the broiler chicken. Appl Environ Microbiol 72:2815–2823
Ben Salah R, Trabelsi I, Ben Mansour R, Lassoued S, Chouayekh H, Bejar S (2012) A new Lactobacillus plantarum strain, TN8, from the gastro intestinal tract of poultry induces high cytokine production. Anaerobe 18:436–444
Patterson JA, Burkholder KM (2003) Application of prebiotics and probiotics in poultry production. Poult Sci 82:627–631
Reid G, Friendship R (2002) Alternatives to antibiotic use: probiotics for the gut. Anim Biotechnol 13:97–112
Svetoch EA, Eruslanov BV, Perelygin VV, Mitsevich EV, Mitsevich IP (2008) Diverse antimicrobial killing by Enterococcus faecium E 50-52 bacteriocin. J Agric Food Chem 56:1942–1948
Lee KW, Park JY, Jeong HR, Heo HJ, Han NS, Kim JH (2012) Probiotic properties of Weissella strains isolated from human faeces. Anaerobe 18:96–102
Dahan S, Dalmasso G, Imbert V, Peyron JF, Rampal P, Czerucka D (2003) Saccharomyces boulardii interferes with enterohemorrhagic Escherichia coli-induced signaling pathways in T84 cells. Infect Immun 71:766–773
Shen X, Falzon M (2005) PTH-related protein enhances LoVo colon cancer cell proliferation, adhesion, and integrin expression. Regul Pept 125:17–27
Srionnual S, Yanagida F, Lin LH, Hsiao KN, Chen YS (2007) Weissellicin 110, a newly discovered bacteriocin from Weissella cibaria 110, isolated from plaa-som, a fermented fish product from Thailand. Appl Environ Microbiol 73:2247–2250
Stern NJ, Svetoch EA, Eruslanov BV, Perelygin VV, Mitsevich EV, Mitsevich IP (2006) Isolation of a Lactobacillus salivarius strain and purification of its bacteriocin, which is inhibitory to Campylobacter jejuni in the chicken gastrointestinal system. Antimicrob Agents Chemother 50:3111–3116
Holck AL, Axelsson L, Huhne K, Krockel L (1994) Purification and cloning of sakacin 674, a bacteriocin from Lactobacillus sake Lb674. FEMS Microbiol Lett 115:143–149
Tichaczek PS, Vogel RF, Hammes WP (1994) Cloning and sequencing of sakP encoding sakacin P, the bacteriocin produced by Lactobacillus sake LTH673. Microbiology 140:361–367
Vaughan A, Eijsink VGH, van Sinderen D, O’Sullivan TF, O’Hanlon K (2001) An analysis of bacteriocins produced by lactic acid bacteria isolated from malted barley. J Appl Microbiol 91:131–138
Urso R, Rantsiou K, Cantoni C, Comi G, Cocolin L (2006) Sequencing and expression analysis of the sakacin P bacteriocin produced by a Lactobacillus sakei strain isolated from naturally fermented sausages. Appl Microbiol Biotechnol 71:480–485
Carvalho KG, Bambirra FHS, Kruger MF, Barbosa MS, Oliveira JS (2010) Antimicrobial compounds produced by Lactobacillus sakei subsp. sakei 2a, a bacteriocinogenic strain isolated from a Brazilian meat product. J Ind Microbiol Biotechnol 37:381–390
Mathiesen G, Huehne K, Kroeckel L, Axelsson L, Eijsink VGH (2005) Characterization of a new bacteriocin operon in sakacin P-producing Lactobacillus sakei, showing strong translational coupling between the bacteriocin and immunity genes. Appl Environ Microbiol 71:3565–3574
Barbosa MS, Todorov SD, Belguesmia Y, Choiset Y, Rabesona H (2014) Purification and characterization of the bacteriocin produced by Lactobacillus sakei MBSa1 isolated from Brazilian salami. J Appl Microbiol 116:1195–1208
Palacios J, Vignolo G, Farías ME, de Ruiz Holgado AP, Oliver G, Sesma F (1999) Purification and amino acid sequence of lactocin 705, a bacteriocin produced by Lactobacillus casei CRL 705. Microbiol Res 154:199–204
Xiraphi N, Georgalaki M, Driessche GV, Devreese B, Beeumen JV (2006) Purification and characterization of curvaticin L442, a bacteriocin produced by Lactobacillus curvatus L442. Antonie Van Leeuwenhoek 89:19–26
Barbosa MS, Todorov SD, Ivanova I, Chobert JM, Haertlé T, de Melo Franco BD (2015) Improving safety of salami by application of bacteriocins produced by an autochthonous Lactobacillus curvatus isolate. Food Microbiol 46:254–262
Casaburi A, Di Martino V, Ferranti P, Picariello L, Villani F (2016) Technological properties and bacteriocins production by Lactobacillus curvatus 54M16 and its use as starter culture for fermented sausage manufacture. Food Control 59:31–45
Rivas FP, Castro MP, Vallejo M, Marguet E, Campos CA (2014) Sakacin Q produced by Lactobacillus curvatus ACU-1: functionality characterization and antilisterial activity on cooked meat surface. Meat Sci 97:475–479
Lozano JCN, Nissen-Meyer J, Sletten K, Pelaz C, Nes IF (1992) Purification and amino acid sequence of a bacteriocin produced by Pediococcus acidilactici. J Gen Microbiol 138:1985–1990
Dortu C, Huch M, Holzapfel WH, Franz CM, Thonart P (2008) Anti-listerial activity of bacteriocin-producing Lactobacillus curvatus CWBI-B28 and Lactobacillus sakei CWBI-B1365 on raw beef and poultry meat. Letters Appl Microbiology 47:581–586
Tichaczek PS, Meyer JN, Nes IF, Vogel RF, Hammes WP (1992) Characterization of the bacteriocins curvacin A from Lactobacillus curvatus LTH 1174 and sakacin P from L. sake LTH. Syst Appl Microbiol 15:460–468
Sudirman I, Mathieu F, Michel M, Lefebvre G (1993) Detection and properties of curvaticin 13, a bacteriocin-like substance produced by Lactobacillus curvatus SB13. Curr Microbiol 27:35–40
Garver KI, Muriana PM (1994) Purification and partial amino acid sequence of curvaticin FS47, a heat-stable bacteriocin produced by Lactobacillus curvatus FS47. Appl Environ Microbiol 60:2191–2195
Ha DM, Cha DS, Han SG (1994) Identification of bacteriocin producing lactic acid bacteria from kimchi and partial characterization of their bacteriocins. J Microbiol Biotechnol 4:305–315
Casla D, Requena T, Gomez R (1996) Antimicrobial activity of lactic acid bacteria isolated from goat milk and artisanal cheeses: characteristics of bacteriocin produced by Lactobacillus curvatus IFPL 105. J Appl Bacteriol 81:35–41
Kim SK, Lee EJ, Park KY, Jun HK (1998) Bacteriocin produced by Lactobacillus curvatus SE1 isolated from kimchi. J Microbiol Biotechnol 8:588–594
Mataragas M, Metaxopoulos J, Drosinos EH (2002) Characterization of two bacteriocins produced by Leuconostoc mesenteroides L124 and Lactobacillus curvatus L442, isolated from dry fermented sausages. World J Microbiol Biotechnol 18:847–856
Ahmadova A, Todorov SD, Hadji-Sfaxi I, Choiset Y, Rabesona H (2013) Antimicrobial and antifungal activities of Lactobacillus curvatus strain isolated from homemade Azerbaijani cheese. Anaerobe 20:42–49
Acknowledgments
This work was supported by the deanship of Research, King Faisal University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All Institutional and National Guidelines for the care and use of chickens were followed.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zommiti, M., Almohammed, H. & Ferchichi, M. Purification and Characterization of a Novel Anti-Campylobacter Bacteriocin Produced by Lactobacillus curvatus DN317. Probiotics & Antimicro. Prot. 8, 191–201 (2016). https://doi.org/10.1007/s12602-016-9237-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-016-9237-7