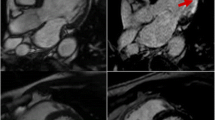
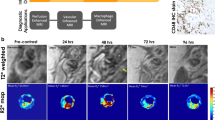
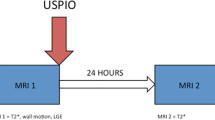
Abstract
Superparamagnetic iron oxide-based contrast agents enhance and complement in vivo magnetic resonance imaging (MRI) by shortening T2 and T2* relaxation times. They are able to highlight areas of cellular inflammation, being detected and engulfed by cells of the reticuloendothelial system, and can be targeted to specific cellular processes or subtypes using antibody or ligand labeling. These agents have been used preclinically for the assessment of cardiac transplant rejection, cardiomyocyte apoptosis, myocardial infarction, myocarditis, and stem and endothelial cell imaging, with clinical applications now emerging. We here review recent studies using iron oxide particles to image cardiac inflammation, and highlight the potential of these agents for future clinical and research applications.



Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Cooper LT, Baughman KL, Feldman AM, Frustaci A, Jessup M, Kuhl U, et al. The role of endomyocardial biopsy in the management of cardiovascular disease. A scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. J Am Coll Cardiol. 2007;50:1914–31.
Wang Y-XJ, Hussain SM, Krestin GP. Superparamagnetic iron oxide contrast agents: physicochemical characteristics and applications in MR imaging. Eur Radiol. 2001;11:2319–31.
Wang Y-XJ. Superparamagnetic iron oxide based MRI contrast agents: current status of clinical application. Quant Imaging Med Surg. 2011;1:35–40.
Allkemper T, Bremer C, Matuszewski L, Ebert W, Reimer P. Contrast-enhanced blood-pool MR angiography with optimized iron oxides: effect of size and dose on vascular contrast enhancement in rabbits. Radiology. 2002;223:432–8.
Mandeville JB, Moore J, Chesler DA, Garrido L, Weissleder R, Weisskoff RM. Dynamic liver imaging with iron oxide agents: effects of size and biodistribution on contrast. Magn Reson Med. 1997;37:885–90.
Hahn PF, Stark DD, Lewis JM, Saini S, Elizondo G, Weissleder R, et al. First clinical trial of a new superparamagnetic iron oxide for use as an oral gastrointestinal contrast agent in MR imaging. Radiology. 1990;175:695–700.
Saini S, Stark DD, Wittenberg J, Brady TJ, Ferrucci JT. Ferrite particles: a superparamagnetic MR contrast agent for the reticuloendothelial system. Radiology. 1987;162:211–6.
Rogers JM, Lewis J, Josephson L. Visualization of superior mesenteric lymph nodes by the combined oral and intravenous administration of the ultrasmall superparamagnetic iron oxide, AMI-227. Magn Reson Imaging. 1994;12:1161–5.
Zimmer C, Weissleder R, O'Connor D, LaPointe L, Brady TJ, Enochs WS. Cerebral iron oxide distribution: in vivo mapping with MR imaging. Radiology. 1995;196:521–7.
Canet E, Revel D, Forrat R, Baldy-Porcher C, de Lorgeril M, Sebbag L, et al. Superparamagnetic iron oxide particles and positive enhancement for myocardial perfusion studies assessed by subsecond T1-weighted MRI. Magn Reson Imaging. 1993;11:1139–45.
Trillaud H, Degrèze P, Mesplède Y, Douws C, Palussière J, Grenier N. Evaluation of experimentally induced renal hypoperfusion using iron oxide particles and fast magnetic resonance imaging. Acad Radiol. 1995;2:293–9.
McAteer MA, Akhtar AM, von Zur Muhlen C, Choudhury RP. An approach to molecular imaging of atherosclerosis, thrombosis, and vascular inflammation using microparticles of iron oxide. Atherosclerosis. 2010;209:18–27.
Wagner S, Schnorr J, Pilgrimm H, Hamm B, Taupitz M. Monomer-coated very small superparamagnetic iron oxide particles as contrast medium for magnetic resonance imaging: preclinical in vivo characterization. Invest Radiol. 2002;37:167–77.
Taylor AM, Panting JR, Keegan J, Gatehouse PD, Amin D, Jhooti P, et al. Safety and preliminary findings with the intravascular contrast agent NC100150 injection for MR coronary angiography. J Magn Reson Imaging. 1999;9:220–7.
Lind K, Kresse M, Debus NP, Müller RH. A novel formulation for superparamagnetic iron oxide (SPIO) particles enhancing MR lymphography: comparison of physicochemical properties and the in vivo behaviour. J Drug Target. 2002;10:221–30.
Clément O, Siauve N, Cuénod CA, Frija G. Liver imaging with ferumoxides (Feridex): fundamentals, controversies, and practical aspects. Top Magn Reson Imaging. 1998;9:167–82.
Bourrinet P, Bengele HH, Bonnemain B, Dencausse A, Idee J-M, Jacobs PM, et al. Preclinical safety and pharmacokinetic profile of ferumoxtran-10, an ultrasmall superparamagnetic iron oxide magnetic resonance contrast agent. Invest Radiol. 2006;41:313–24.
Yang Y, Yang Y, Yanasak N, Schumacher A, Hu TCC. Temporal and noninvasive monitoring of inflammatory—cell infiltration to myocardial infarction sites using micrometer—sized iron oxide particles. Magn Reson Med. 2010;63:33–40.
Ye Q, Wu YL, Foley LM, Hitchens TK, Eytan DF, Shirwan H, et al. Longitudinal tracking of recipient macrophages in a rat chronic cardiac allograft rejection model with noninvasive magnetic resonance imaging using micrometer-sized paramagnetic iron oxide particles. Circulation. 2008;118:149–56.
Briley-Saebo KC, Johansson LO, Hustvedt SO, Haldorsen AG, Bjørnerud A, Fayad ZA, et al. Clearance of iron oxide particles in rat liver: effect of hydrated particle size and coating material on liver metabolism. Invest Radiol. 2006;41:560–71.
Shapiro EM, Sharer K, Skrtic S, Koretsky AP. In vivo detection of single cells by MRI. Magn Reson Med. 2006;55:242–9.
Shapiro EM, Skrtic S, Sharer K, Hill JM, Dunbar CE, Koretsky AP. MRI detection of single particles for cellular imaging. Proc Natl Acad Sci U S A. 2004;101:10901–6.
Hertz MI, Aurora P, Christie JD, Dobbels F, Edwards LB, Kirk R, et al. Scientific Registry of the International Society for Heart and Lung Transplantation: introduction to the 2009 Annual Reports. J Heart Lung Transplant. 2009;28:989–92.
Stewart S, Winters GL, Fishbein MC, Tazelaar HD, Kobashigawa J, Abrams J, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24:1710–20.
Kanno S, Wu Y-JL, Lee PC, Dodd SJ, Williams M, Griffith BP, et al. Macrophage accumulation associated with rat cardiac allograft rejection detected by magnetic resonance imaging with ultrasmall superparamagnetic iron oxide particles. Circulation. 2001;104:934–8.
Wu YL, Ye Q, Sato K, Foley LM, Hitchens TK, Ho C. Noninvasive evaluation of cardiac allograft rejection by cellular and functional cardiac magnetic resonance. JACC Cardiovasc Imaging. 2009;2:731–41.
Guo Y, Chen W, Wang W, Shen J, Guo R, Gong F, et al. Simultaneous diagnosis and gene therapy of immuno-rejection in rat allogeneic heart transplantation model using a t-cell-targeted theranostic nanosystem. ACS Nano. 2012;121204131551005. Novel “theranostic” nanosystem allowing diagnosis and treatment (via gene therapy) of allogeneic cardiac transplantation rejection in rats.
Narula J, Acio ER, Narula N, Samuels LE, Fyfe B, Wood D, et al. Annexin-V imaging for noninvasive detection of cardiac allograft rejection. Nat Med. 2001;7:1347–52. doi:10.1038/nm1101-1241 [Pub online: Nov 1, 2001].
Dash R, Chung J, Chan T, Yamada M, Barral J, Nishimura D, et al. A molecular MRI probe to detect treatment of cardiac apoptosis in vivo. Magn Reson Med. 2011;66:1152–62.
Wunderbaldinger P, Josephson L, Weissleder R. Crosslinked iron oxides (CLIO): a new platform for the development of targeted MR contrast agents. Acad Radiol. 2002;9 Suppl 2:S304–6.
Schellenberger EA, Högemann D, Josephson L, Weissleder R. Annexin V-CLIO: a nanoparticle for detecting apoptosis by MRI. Acad Radiol. 2002;9 Suppl 2:S310–1.
Sosnovik DE, Schellenberger EA, Nahrendorf M, Novikov MS, Matsui T, Dai G, et al. Magnetic resonance imaging of cardiomyocyte apoptosis with a novel magneto—optical nanoparticle. Magn Reson Med. 2005;54:718–24.
Sosnovik DE, Garanger E, Aikawa E, Nahrendorf M, Figuiredo J-L, Dai G, et al. Molecular MRI of cardiomyocyte apoptosis with simultaneous delayed-enhancement MRI distinguishes apoptotic and necrotic myocytes in vivo potential for midmyocardial salvage in acute ischemia. Circ Cardiovasc Imaging. 2009;2:460–7.
Yang Y, Liu J, Yang Y, Cho SH, Hu TCC. Assessment of cell infiltration in myocardial infarction: a dose-dependent study using micrometer-sized iron oxide particles. Magn Reson Med. 2011;66:1353–61.
Alam SR, Shah ASV, Richards J, Lang NN, Barnes G, Joshi N, et al. Ultrasmall superparamagnetic particles of iron oxide in patients with acute myocardial infarction: early clinical experience. Circ Cardiovasc Imaging. 2012;5:559–65. First human studies evaluating uptake of iron nanoparticles after myocardial infarction.
Yilmaz A, Dengler MA, van der Kuip H, Yildiz H, Rosch S, Klumpp S, et al. Imaging of myocardial infarction using ultrasmall superparamagnetic iron oxide nanoparticles: a human study using a multi-parametric cardiovascular magnetic resonance imaging approach. Eur Heart J. 2013;34:462–75. First human studies evaluating uptake of iron nanoparticles after myocardial infarction.
Grieve SM, Lønborg J, Mazhar J, Tan TC, Ho E, Liu C-C, et al. Cardiac magnetic resonance imaging of rapid VCAM-1 up-regulation in myocardial ischemia–reperfusion injury. Eur Biophys J. 2012;42:61–70.
Ruparelia N, Digby JE, Jefferson A, Medway DJ, Neubauer S, Lygate CA, et al. Myocardial infarction causes inflammation and leukocyte recruitment at remote sites in the myocardium and in the renal glomerulus. Inflamm Res. 2013;62:515–25.
Moon H, Park HE, Kang J, Lee H, Cheong C, Lim YT, et al. Noninvasive assessment of myocardial inflammation by cardiovascular magnetic resonance in a rat model of experimental autoimmune myocarditis. Circulation. 2012;125:2603–12.
Kraitchman DL. In vivo magnetic resonance imaging of mesenchymal stem cells in myocardial infarction. Circulation. 2003;107:2290–3.
Lu M, Zhao S, Liu Q, Jiang S, Song P, Qian H, et al. Transplantation with autologous mesenchymal stem cells after acute myocardial infarction evaluated by magnetic resonance imaging: an experimental study. J Thorac Imaging. 2012;27:125–35.
Chung J, Kee K, Barral JK, Dash R, Kosuge H, Wang X, et al. In vivo molecular MRI of cell survival and teratoma formation following embryonic stem cell transplantation into the injured murine myocardium. Magn Reson Med. 2011;66:1374–81. Imaging of proliferation and teratoma formation after embryonic stem cell transplantation. A reporter gene expressing antigen on the surface of stem cells allowed quantification of viability and proliferation of stem cells whilst monitoring for teratoma formation.
Dall’Armellina E, Lygate CA, Mcateer M. Ex vivo and in vivo MR imaging of ischemia reperfusion injury in mouse hearts using microparticles of iron oxide targeting VCAM-1. Proceedings of the 18th Annual Meeting ISMRM, Stockholm, Sweden, 2010;3637.
Gabrielsen A, Lawler PR, Yongzhong W, Steinbrüchel D, Blagoja D, Paulsson-Berne G, et al. Gene expression signals involved in ischemic injury, extracellular matrix composition and fibrosis defined by global mRNA profiling of the human left ventricular myocardium. J Mol Cell Cardiol. 2007;42:870–83.
Heesakkers RAM, Jager GJ, Hövels AM, de Hoop B, van den Bosch HCM, Raat F, et al. Prostate cancer: detection of lymph node metastases outside the routine surgical area with ferumoxtran-10-enhanced MR imaging. Radiology. 2009;251:408–14.
Funding
The British Heart Foundation (BHF) and Chief Scientist Office (CSO) have provided grants supporting work in this field at our center carried out by CS, JR and DEN. Work currently carried out by CS and DEN is supported by grants from the the BHF (FS/12/83/29781) and the CSO (ETM/266). JR was supported by a BHF Clinical PhD Training Fellowship (FS/07/060). DEN is supported by the British Heart Foundation (CH/09/002).
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Colin G Stirrat, David E Newby, Jennifer MJ Robson, and Maurits A Jansen declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Molecular Imaging
Rights and permissions
About this article
Cite this article
Stirrat, C.G., Newby, D.E., Robson, J.M.J. et al. The Use of Superparamagnetic Iron Oxide Nanoparticles to Assess Cardiac Inflammation. Curr Cardiovasc Imaging Rep 7, 9263 (2014). https://doi.org/10.1007/s12410-014-9263-3
Published:
DOI: https://doi.org/10.1007/s12410-014-9263-3