Abstract
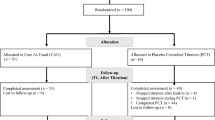
Several extended-release methylphenidate medications are available for treatment of children with ADHD. Pharmacokinetic investigations suggest that the serum levels of methylphenidate are partially altered when the medication is taken without breakfast. Clinical data comparing different breakfast situations are missing. In this study, different breakfast compositions and their influence on treatment with Ritalin LA are investigated. A total of 150 patients were enrolled in a rater-blinded, randomized crossover trial that compared a minimal breakfast with a standard breakfast in patients under stable treatment with Ritalin LA. Ratings for clinical efficacy were carried out after 1 week by teachers and parents (FBB-ADHS), as well as physicians (CGI). Additionally, a math test was administered to the patients. Of the total patients, 144 finished the trial with a breakfast compliance of 93%. All of the clinical rating scales showed consistently no difference between the two breakfast conditions. Non-inferiority of minimal breakfast versus standard breakfast was shown to be statistically significant (FBB-AHDSTeacher: 0.97 with minimal breakfast, 1.01 with standard breakfast, P < 0.0001). The clinical efficacy of Ritalin LA is not influenced by breakfast and works independently of food intake.

Similar content being viewed by others
References
Banaschewski T, Coghill D, Santosh P, Zuddas A, Asherson P, Buitelaar J, Danckaerts M, Doepfner M, Faraone SV, Rothenberger A, Sergeant J, Steinhausen HC, Sonuga-Barke EJS, Taylor E (2006) Long acting medications for the hyperkinetic disorders–A systematic review and European treatment guideline. Eur Child Adolesc Psychiatry 15(8):476–495
Bruehl B, Doepfner M, Lehmkuhl G (2000) Der Fremdbeurteilungsbogen für hyperkinetische Störungen (FBB-HKS)–Prävalenz hyperkinetischer Störungen im Elternurteil und psychometrische Kriterien. Kindheit und Entwicklung 9(2):116–126
Deutsche Gesellschaft für Ernährung (2000) Referenzwerte für die Nährstoffzufuhr. Umschau/Braus, Frankfurt/Min
Doepfner M, Lehmkuhl G, Steinhausen HC (2006) KIDS (Kinder-Diagnostik-System)-AHDS. Hogrefe Verlag, Göttingen
Guy W (1976) Clinical Global Impression. In: ECDEU Assessment manual for psychopharmacology. US department of health, education and welfare. Rev. Rockville, Maryland
Haessler F, Tracik F, Dietrich H, Stammer H, Klatt J (2008) A pharmacokinetic study of two modified-release methylphenidate formulations under different food conditions in healthy volunteers. Int J Clin Pharmacol Ther 46(9):466–476
Lee L, Kepple J, Wang Y, Freestone S, Bakhtiar R, Wang Y, Hossain M (2003) Bioavailability of modified-release methylphenidate: influence of high-fat breakfast when administered intact and when capsule content sprinkled on applesauce. Biopharm Drug Dispos 24:233–243
Marcus SC, Wan GJ, Kemner JE, Olfson M (2006) Continuity of methylphenidate treatment for attention-deficity hyperactivity disorder. Arch Pediatr Adolesc Med 159:572–578
Medice Arzneimittel Pütter GmbH & Co. KG (2008) Medikinet® retard 10 mg/20 mg/30 mg/40 mg. Zusammenfassung der Merkmale des Arzneimittels (Product information)
Schlack R, Hölling H, Kurth BM, Huss M (2007) The prevalence of ADHD among children and adolescents in Germany. Initial results from the German Health Interview and Examination Survey for Children and Adolescents [KiGGS]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 50(5–6):827–835
Swanson JM, Wigal SB, Udrea D, Lerner M, Agler D, Flynn D, Fineberg E, Davies M, Kardatzke D, Ram A, Gupta S (1998) Objective and subjective measurements of the pharmacodynamic effects of Adderall in the treatment of children with ADHD in a controlled laboratory classroom setting. Psychopharmacol Bull 34(1):55–61
Wolraich ML, McGuinn L, Doffing M (2007) Treatment of attention deficit hyperactivity disorder in children and adolescents–safety considerations. Drug Safety 30(1):17–26
Acknowledgments
The authors would like to thank all investigators and co-investigators from the 13 participating study sites for the Breakfast Study Group as well as Mrs. Clement for creating the different breakfast schedules. This study was sponsored by Novartis Pharma GmbH, Germany.
Conflict of interest
Eberhard Schulz: received grants from BMBF, Eli Lilly, Janssen-Cilag, Novartis, Pfizer and Shire.
Christian Fleischhaker received grants from Bristol-Myers Squibb (Advisory Board).
Frank Haessler M.D.:
Advisory Board: Eli Lilly GmbH Germany, Janssen-Cilag
Research Support: Eli Lilly GmbH Germany, Janssen-Cilag, Novartis Pharmaceuticals, Bayer Vital
Travel Grants: Novartis Pharmaceuticals, AstraZeneca Pharmaceutical, UCB
Consulting Fees: Janssen-Cilag, Novartis Pharmaceuticals, UCB
Honoraria: UCB
Andreas Warnke received grants for scientific presentations, pharmacological research and scientific meetings from Astra-Zeneca, Eli Lilly, Janssen-Cilag, Medice, Novartis, Sanofi, Shire, Solvay and UCB.
Klaus Hennighausen, Martin Linder and Kirsten Stollhoff have received financial compensation from the sponsor for participation in this trial. Monika Baier, Ferenc Tracik and Jan Klatt are employees of Novartis Pharma GmbH, Germany.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study is conducted on behalf of the Breakfast Study group.
Rights and permissions
About this article
Cite this article
Schulz, E., Fleischhaker, C., Hennighausen, K. et al. A randomized, rater-blinded, crossover study comparing the clinical efficacy of Ritalin® LA (methylphenidate) treatment in children with attention-deficit hyperactivity disorder under different breakfast conditions over 2 weeks. ADHD Atten Def Hyp Disord 2, 133–138 (2010). https://doi.org/10.1007/s12402-010-0031-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12402-010-0031-1