Abstract
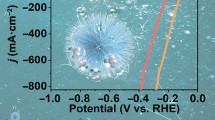
The development of noble-metal-free electrocatalysts for water splitting is indispensable for the efficient production of hydrogen fuel. Herein, a Co-doped Ni-Mo phosphide nanorod arrays fabricated on porous Ni foam was shown to be an efficient binder-free electrocatalyst for water splitting. This catalyst featured exceptional activity, exhibiting an overpotential of 29 mV at a current density of 10 mA·cm−2 for the hydrogen evolution reaction, whereas the corresponding precatalyst exhibited an overpotential of 314 mV at a current density of 50 mA·cm−2 for the oxygen evolution reaction. The achieved electrocatalytic performance provided access to a simple water splitting system, affording a current density of 10 mA·cm−2 at 1.47 V in 1 M KOH electrolyte. Density functional theory results indicated that Co doping and phosphorization were responsible for the high electrocatalytic performance. Thus, this work paves the way for the development of novel noble-metal-free electrocatalysts for practical H2 production via water splitting.

Similar content being viewed by others
References
Ming, M.; Zhang, Y.; He, C.; Zhao, L.; Niu, S.; Fang, G. Y.; Hu, J. S. Room-temperature sustainable synthesis of selected platinum group metal (PGM = Ir, Rh, and Ru) nanocatalysts well-dispersed on porous carbon for efficient hydrogen evolution and oxidation. Small 2019, 15, 1903057.
Cheng, H.; Su, Y. Z.; Kuang, P. Y.; Chen, G. F.; Liu, Z. Q. Hierarchical NiCo2O4 nanosheet-decorated carbon nanotubes towards highly efficient electrocatalyst for water oxidation. J. Mater. Chem. A 2015, 3, 19314–19321.
Lu, W. B.; Liu, T. T.; Xie, L. S.; Tang, C.; Liu, D. N.; Hao, S.; Qu, F. L.; Du, G.; Ma, Y. J.; Asiri, A. M.; et al. In situ derived Co-B nanoarray: A high-efficiency and durable 3D bifunctional electrocatalyst for overall alkaline water splitting. Small 2017, 13, 1700805.
Faber, M. S.; Jin, S. Earth-abundant inorganic electrocatalysts and their nanostructures for energy conversion applications. Energy Environ. Sci. 2014, 7, 3519–3542.
Subbaraman, R.; Tripkovic, D.; Strmcnik, D.; Chang, K. C.; Uchimura, M.; Paulikas, A. P.; Stamenkovic, V.; Markovic, N. M. Enhancing hydrogen evolution activity in water splitting by tailoring Li+-Ni(OH)2-Pt interfaces. Science 2011, 334, 1256–1260.
Yang, L.; Guo, Z. L.; Huang, J.; Xi, Y. N.; Gao, R. J.; Su, G.; Wang, W.; Cao, L. X.; Dong, B. H. Vertical growth of 2D amorphous FePO4 nanosheet on Ni foam: Outer and inner structural design for superior water splitting. Adv. Mater. 2017, 29, 1704574.
Han, L.; Dong, S. J.; Wang, E. K. Transition-metal (Co, Ni, and Fe)-based electrocatalysts for the water oxidation reaction. Adv. Mater. 2016, 28, 9266–9291.
Anantharaj, S.; Ede, S. R.; Sakthikumar, K.; Karthick, K.; Mishra, S.; Kundu, S. Recent trends and perspectives in electrochemical water splitting with an emphasis on sulfide, selenide, and phosphide catalysts of Fe, Co, and Ni: A review. ACS Catal. 2016, 6, 8069–8097.
Yan, Y.; Xia, B. Y.; Zhao, B.; Wang, X. A review on noble-metal-free bifunctional heterogeneous catalysts for overall electrochemical water splitting. J. Mater. Chem. A 2016, 4, 17587–17603.
Sun, H. M.; Yan, Z. H.; Liu, F. M.; Xu, W. C.; Cheng, F. Y.; Chen, J. Self-supported transition-metal-based electrocatalysts for hydrogen and oxygen evolution. Adv. Mater. 2020, 32, 1806326.
Faber, M. S.; Dziedzic, R.; Lukowski, M. A.; Kaiser, N. S.; Ding, Q.; Jin, S. High-performance electrocatalysis using metallic cobalt pyrite (CoS2) micro-and nanostructures. J. Am. Chem. Soc. 2014, 136, 10053–10061.
Feng, L. L.; Yu, G. T.; Wu, Y. Y.; Li, G. D.; Li, H.; Sun, Y. H.; Asefa, T.; Chen, W.; Zou, X. X. High-index faceted Ni3S2nanosheet arrays as highly active and ultrastable electrocatalysts for water splitting. J. Am. Chem. Soc. 2015, 137, 14023–14026.
Yu, J. Y.; Li, G. X.; Liu, H.; Zhao, L. L.; Wang, A. Z.; Liu, Z.; Li, H. D.; Liu, H.; Hu, Y. Y.; Zhou, W. J. Ru-Ru2PΦNPC and NPC@RuO2 synthesized via environment-friendly and solid-phase phosphating process by saccharomycetes as N/P sources and carbon template for overall water splitting in acid electrolyte. Adv. Funct. Mater. 2019, 29, 1901154.
Chen, Y. K.; Yu, J. Y.; Jia, J.; Liu, F.; Zhang, Y. W.; Xiong, G. W.; Zhang, R. T.; Yang, R. Q.; Sun, D. H.; Liu, H. et al. Metallic Ni3Mo3N porous microrods with abundant catalytic sites as efficient electrocatalyst for large current density and superstability of hydrogen evolution reaction and water splitting. Appl. Catal. B: Environ. 2020, 272, 118956.
Shan, J. Q.; Ling, T.; Davey, K.; Zheng, Y.; Qiao, S. Z. Transition-metal-doped RuIr bifunctional nanocrystals for overall water splitting in acidic environments. Adv. Mater. 2019, 31, 1900510.
Xie, L. S.; Qu, F. L.; Liu, Z. A.; Ren, X.; Hao, S.; Ge, R. X.; Du, G.; Asiri, A. M.; Sun, X. P.; Chen, L. In situ formation of a 3D core/shell structured Ni3N@Ni-Bi nanosheet array: An efficient non-noble-metal bifunctional electrocatalyst toward full water splitting under near-neutral conditions. J. Mater. Chem. A 2017, 5, 7806–7810.
Xiong, Q. Z.; Zhang, X.; Wang, H. J.; Liu, G. Q.; Wang, G. Z.; Zhang, H. M.; Zhao, H. J. One-step synthesis of cobalt-doped MoS2 nanosheets as bifunctional electrocatalysts for overall water splitting under both acidic and alkaline conditions. Chem. Commun. 2018, 54, 3859–3862.
Wu, Z. C.; Zou, Z. X.; Huang, J. S.; Gao, F. Fe-doped NiO mesoporous nanosheets array for highly efficient overall water splitting. J. Catal. 2018, 358, 243–252.
Yuan, C. Z.; Zhong, S. L.; Jiang, Y. F.; Yang, Z. K.; Zhao, Z. W.; Zhao, S. J.; Jiang, N.; Xu, A. W. Direct growth of cobalt-rich cobalt phosphide catalysts on cobalt foil: An efficient and self-supported bifunctional electrode for overall water splitting in alkaline media. J. Mater. Chem. A 2017, 5, 10561–10566.
Shan, X. Y.; Liu, J.; Mu, H. R.; Xiao, Y.; Mei, B. B.; Liu, W. G.; Lin, G.; Jiang, Z.; Wen, L. P.; Jiang, L. An engineered superhydrophilic/superaerophobic electrocatalyst composed of the supported CoMoSx chalcogel for overall water splitting. Angew. Chem., Int. Ed. 2020, 59, 1659–1665.
Zhu, X. Y.; Zhang, X. P.; Huang, L.; Liu, Y. Q.; Zhang, H.; Dong, S. J. Cobalt doped β-molybdenum carbide nanoparticles encapsulated within nitrogen-doped carbon for oxygen evolution. Chem. Commun. 2019, 55, 9995–9998.
Li, G. X.; Wang, J. G.; Yu, J. Y.; Liu, H.; Cao, Q.; Du, J. L.; Zhao, L. L.; Jia, J.; Liu, H.; Zhou, W. J. Ni-Ni3P nanoparticles embedded into N, P-doped carbon on 3D graphene frameworks via in situ phosphatization of saccharomycetes with multifunctional electrodes for electrocatalytic hydrogen production and anodic degradation. Appl. Catal. B: Environ. 2020, 261, 118147.
Yu, X. B.; Zhang, S.; Li, C. Y.; Zhu, C. L.; Chen, Y. J.; Gao, P.; Qi, L. H.; Zhang, X. T. Hollow CoP nanopaticle/N-doped graphene hybrids as highly active and stable bifunctional catalysts for full water splitting. Nanoscale 2016, 8, 10902–10907.
Yin, Z. X.; Zhu, C. L.; Li, C. Y.; Zhang, S.; Zhang, X. T.; Chen, Y. J. Hierarchical nickel-cobalt phosphide yolk-shell spheres as highly active and stable bifunctional electrocatalysts for overall water splitting. Nanoscale 2016, 8, 19129–19138.
Liu, T. T.; Xie, L. S.; Yang, J. H.; Kong, R. M.; Du, G.; Asiri, A. M.; Sun, X. P.; Chen, L. Self-standing CoP nanosheets array: A three-dimensional bifunctional catalyst electrode for overall water splitting in both neutral and alkaline media. ChemElectroChem 2017, 4, 1840–1845.
Hu, E. L.; Feng, Y. F.; Nai, J. W.; Zhao, D. A.; Hu, Y.; Lou, X. W. Construction of hierarchical Ni-Co-P hollow nanobricks with oriented nanosheets for efficient overall water splitting. Energy Environ. Sci. 2018, 11, 872–880.
Liu, T. T.; Liu, D. N.; Qu, F. L.; Wang, D. X.; Zhang, L.; Ge, R. X.; Hao, S.; Ma, Y. J.; Du, G.; Asiri, A. M. et al. Enhanced electrocatalysis for energy-efficient hydrogen production over CoP catalyst with nonelectroactive Zn as a promoter. Adv. Energy Mater. 2017, 7, 1700020.
Liu, P.; Rodriguez, J. A. Catalysts for hydrogen evolution from the [NiFe] hydrogenase to the Ni2P(001) surface: The importance of ensemble effect. J. Am. Chem. Soc. 2005, 127, 14871–14878.
Ledendecker, M.; Calderón, S. K.; Papp, C.; Steinrück, H. P.; Antonietti, M.; Shalom, M. The synthesis of nanostructured Ni5P4 films and their use as a non-noble bifunctional electrocatalyst for full water splitting. Angew. Chem., Int. Ed. 2015, 54, 12361–12365.
Li, K.; Rakov, D.; Zhang, W.; Xu, P. Improving the intrinsic electrocatalytic hydrogen evolution activity of few-layer NiPS3 by cobalt doping. Chem. Commun. 2017, 53, 8199–8202.
Fu, S. F.; Zhu, C. Z.; Song, J. H.; Engelhard, M. H.; Li, X. L.; Du, D.; Lin, Y. H. Highly ordered mesoporous bimetallic phosphides as efficient oxygen evolution electrocatalysts. ACS Energy Lett. 2016, 1, 792–796.
Liang, X.; Zheng, B. X.; Chen, L. G.; Zhang, J. T.; Zhuang, Z. B.; Chen, B. H. MOF-derived formation of Ni2P-CoP bimetallic phosphides with strong interfacial effect toward electrocatalytic water splitting. ACS Appl. Mater. Interfaces 2017, 9, 23222–23229.
Wang, Y.; Sun, Y.; Yan, F.; Zhu, C. L.; Gao, P.; Zhang, X. T.; Chen, Y. J. Self-supported NiMo-based nanowire arrays as bifunctional electrocatalysts for full water splitting. J. Mater. Chem. A 2018, 6, 8479–8487.
McKone, J. R.; Sadtler, B. F.; Werlang, C. A.; Lewis, N. S.; Gray, H. B. Ni-Mo nanopowders for efficient electrochemical hydrogen evolution. ACS Catal. 2013, 3, 166–169.
Wang, Y.; Wang, M. Y.; Zhang, Z. S.; Wang, Q.; Jiang, Z.; Lucero, M.; Zhang, X.; Li, X. X.; Gu, M.; Feng, Z. X. et al. Phthalocyanine precursors to construct atomically dispersed iron electrocatalysts. ACS Catal. 2019, 9, 6252–6261.
Zhang, Y.; Liu, Y. W.; Ma, M.; Ren, X.; Liu, Z. A.; Du, G.; Asiri, A. M.; Sun, X. P. A Mn-doped Ni2P nanosheet array: An efficient and durable hydrogen evolution reaction electrocatalyst in alkaline media. Chem. Commun. 2017, 53, 11048–11051.
Du, Z. H.; Zhao, H. L.; Yi, S.; Xia, Q.; Gong, Y.; Zhang, Y.; Cheng, X.; Li, Y.; Gu, L.; Świerczek, K. High-performance anode material Sr2FeMo0.65Ni0.35O6−δ with in situ exsolved nanoparticle catalyst. ACS Nano 2016, 10, 8660–8669.
Wei, X. J.; Zhang, Y. H.; He, H. C.; Gao, D.; Hu, J. R.; Peng, H. R.; Peng, L.; Xiao, S. H.; Xiao, P. Carbon-incorporated NiO/Co3O4 concave surface microcubes derived from a MOF precursor for overall water splitting. Chem. Commun. 2019, 55, 6515–6518.
Moosavifard, S. E.; Fani, S.; Rahmanian, M. Hierarchical CuCo2S4 hollow nanoneedle arrays as novel binder-free electrodes for highperformance asymmetric supercapacitors. Chem. Commum. 2016, 52, 4517–4520.
He, L. H.; Zhang, S.; Ji, H. F.; Wang, M. H.; Peng, D. L.; Yan, F. F.; Fang, S. M.; Zhang, H. Z.; Jia, C. X.; Zhang, Z. H. Protein-templated cobaltous phosphate nanocomposites for the highly sensitive and selective detection of platelet-derived growth factor-BB. Biosens. Bioelectron. 2016, 79, 553–560.
Zhang, R.; Wang, X. X.; Yu, S. J.; Wen, T.; Zhu, X. W.; Yang, F. X.; Sun, X. N.; Wang, X. K.; Hu, W. P. Ternary NiCo2Px nanowires as pH-universal electrocatalysts for highly efficient hydrogen evolution reaction. Adv. Mater. 2017, 29, 1605502.
Gong, Y. Q.; Xu, Z. F.; Pan, H. L.; Lin, Y.; Yang, Z.; Wang, J. L. A 3D well-matched electrode pair of Ni-Co-S//Ni-Co-P nanoarrays grown on nickel foam as a high-performance electrocatalyst for water splitting. J. Mater. Chem. A 2018, 6, 12506–12514.
Deng, Y. Q.; Yang, L. J.; Wang, Y. K.; Zeng, L. L.; Yu, J. Y.; Chen, B.; Zhang, X. L.; Zhou, W. J. Ruthenium nanoclusters anchored on cobalt phosphide hollow microspheres by green phosphating process for full water splitting in acidic electrolyte. Chin. Chem. Lett., in press, DOI: https://doi.org/10.1016/j.cclet.2020.03.076.
Zhao, Y. Q.; Jin, B.; Vasileff, A.; Jiao, Y.; Qiao, S. Z. Interfacial nickel nitride/sulfide as a bifunctional electrode for highly efficient overall water/seawater electrolysis. J. Mater. Chem. A 2019, 7, 8117–8121.
Chen, P. Z.; Xu, K.; Tao, S.; Zhou, T. P.; Tong, Y.; Ding, H.; Zhang, L. D.; Chu, W. S.; Wu, C. Z.; Xie, Y. Phase-transformation engineering in cobalt diselenide realizing enhanced catalytic activity for hydrogen evolution in an alkaline medium. Adv. Mater. 2016, 28, 7527–7532.
Zheng, Y.; Jiao, Y.; Zhu, Y. H.; Li, L. H.; Han, Y.; Chen, Y.; Du, A. J.; Jaroniec, M.; Qiao, S. Z. Hydrogen evolution by a metal-free electrocatalyst. Nat. Commun. 2014, 5, 3783.
Deng, R.; Chen, Q.; Luo, Q.; Zhou, L. X.; Wang, Y.; Zhang, Y.; Fan, G. Y. Salt template-assisted in situ construction of Ru nanoclusters and porous carbon: Excellent catalysts toward hydrogen evolution, ammonia-borane hydrolysis, and 4-nitrophenol reduction. Green Chem. 2020, 22, 835–842.
Jin, S. Are metal chalcogenides, nitrides, and phosphides oxygen evolution catalysts or bifunctional catalysts? ACS Energy Lett. 2017, 2, 1937–1938.
Luo, X.; Ji, P. X.; Wang, P. Y.; Cheng, R. L.; Chen, D.; Lin, C.; Zhang, J. N.; He, J. W.; Shi, Z. H.; Li, N. et al. Interface engineering of hierarchical branched Mo-doped Ni3S2/Nix Py hollow heterostructure nanorods for efficient overall water splitting. Adv. Energy Mater. 2020, 10, 1903891.
Tang, C.; Cheng, N. Y.; Pu, Z. H.; Xing, W.; Sun, X. P. NiSe nanowire film supported on nickel foam: An efficient and stable 3D bifunctional electrode for full water splitting. Angew. Chem., Int. Ed. 2015, 54, 9351–9355.
Zeng, L. Y.; Sun, K. A.; Wang, X. B.; Liu, Y. Q.; Pan, Y.; Liu, Z.; Cao, D. W.; Song, Y.; Liu, S. H.; Liu, C. G. Three-dimensional-networked Ni2P/Ni3S2 heteronanoflake arrays for highly enhanced electrochemical overall-water-splitting activity. Nano Energy 2018, 51, 26–36.
Stern, L. A.; Feng, L. G.; Song, F.; Hu, X. L. Ni2P as a Janus catalyst for water splitting: The oxygen evolution activity of Ni2P nanoparticles. Energy Environ. Sci. 2015, 8, 2347–2351.
Sun, Y. Q.; Hang, L. F.; Shen, Q.; Zhang, T.; Li, H. L.; Zhang, X. M.; Lyu, X. J.; Li, Y. Mo doped Ni2P nanowire arrays: An efficient electrocatalyst for the hydrogen evolution reaction with enhanced activity at all pH values. Nanoscale 2017, 9, 16674–16679.
Lukowski, M. A.; Daniel, A. S.; English, C. R.; Meng, F.; Forticaux, A.; Hamers, R. J.; Jin, S. Highly active hydrogen evolution catalysis from metallic WS2nanosheets. Energy Environ. Sci. 2014, 7, 2608–2613.
Liang, H. F.; Gandi, A. N.; Anjum, D. H.; Wang, X. B.; Schwingenschlögl, U.; Alshareef, H. N. Plasma-assisted synthesis of NiCoP for efficient overall water splitting. Nano Lett. 2016, 16, 7718–7725.
Du, C.; Yang, L.; Yang, F. L.; Cheng, G. Z.; Luo, W. Nest-like NiCoP for highly efficient overall water splitting. ACS Catal. 2017, 7, 4131–4137.
Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186.
Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50.
Perdew, J. P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 51827901). We would like to thank the Institute of New Energy and Low-Carbon Technology, Sichuan University, for XRD analysis work. We also thank Clean Energy Research Institute for the support.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
12274_2020_3010_MOESM1_ESM.pdf
Noble-metal-free catalyst with enhanced hydrogen evolution reaction activity based on granulated Co-doped Ni-Mo phosphide nanorod arrays
Rights and permissions
About this article
Cite this article
Xie, H., Lan, C., Chen, B. et al. Noble-metal-free catalyst with enhanced hydrogen evolution reaction activity based on granulated Co-doped Ni-Mo phosphide nanorod arrays. Nano Res. 13, 3321–3329 (2020). https://doi.org/10.1007/s12274-020-3010-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-020-3010-7