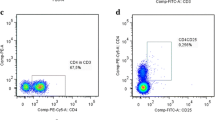
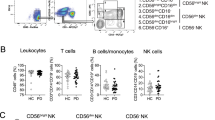
Abstract
In this study, we conducted a clinical analysis of lymphocyte subtypes in 268 patients with Parkinson’s disease (PD) to assess their clinical impact as a potential marker of advanced PD in Chinese patients. The participants comprised 268 sporadic PD patients and 268 healthy controls. The numbers of natural killer (NK) cells and CD3+, CD3+CD4+, CD3+CD8+, and CD19+ lymphocytes from peripheral blood were determined by immunostaining and flow cytometric analysis and the percentages of these CD+ T cells were calculated. The ratio of regulatory T (Treg)/helper T 17 (Th17) lymphocytes from 64 PD patients and 46 controls was determined by flow cytometric analysis. The results showed that the percentage of NK cells was higher in advanced PD patients than in controls (22.92% ± 10.08% versus 19.76% ± 10.09%, P = 0.006), while CD3+ T cells are decreased (62.93% ± 9.27% versus 65.75% ± 9.13%, P = 0.005). The percentage of CD19+ B cells in male patients was lower (P = 0.021) than in female patients, whereas NK cells were increased (P < 0.0001). The scores on the Unified Parkinson’s Disease Rating Scale (UPDRS) and the Non-Motor Symptoms Scale in late-onset PD patients were significantly higher than those in early-onset patients (P = 0.024 and P = 0.007, respectively). The percentage of CD19+ B cells in patients with UPDRS scores >24 was lower than in those with scores <24 (10.17% ± 4.19% versus 12.22% ± 5.39%, P = 0.009). In addition, the Treg/Th17 ratio in female patients was higher than that in female controls (13.88 ± 6.32 versus 9.94 ± 4.06, P = 0.042). These results suggest that the percentages of NK cells, CD3+ T cells, and CD19+ B cells along with the Treg/Th17 ratio in peripheral blood may be used to predict the risk of PD in Chinese individuals and provide fresh avenues for novel diagnostic biomarkers and therapeutic designs.


Similar content being viewed by others

References
Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron 2003, 39: 889–909.
Connolly BS, Lang AE. Pharmacological treatment of Parkinson disease: a review. Jama 2014, 311: 1670–1683.
Dorsey ER, Constantinescu R, Thompson JP, Biglan KM, Holloway RG, Kieburtz K, et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 2007, 68: 384–386.
Lorincz MT. Clinical implications of Parkinson’s disease genetics. Semin Neurol 2006, 26: 492–498.
Noelker C, Morel L, Osterloh A, Alvarez-Fischer D, Lescot T, Breloer M, et al. Heat shock protein 60: an endogenous inducer of dopaminergic cell death in Parkinson disease. J Neuroinflammation 2014, 11: 86.
More SV, Kumar H, Kim IS, Song SY, Choi DK. Cellular and molecular mediators of neuroinflammation in the pathogenesis of Parkinson’s disease. Mediators Inflamm 2013, 2013: 952375.
Kannarkat GT, Boss JM, Tansey MG. The role of innate and adaptive immunity in Parkinson’s disease. J Parkinsons Dis 2013, 3: 493–514.
Beers DR, Henkel JS, Zhao W, Wang J, Huang A, Wen S, et al. Endogenous regulatory T lymphocytes ameliorate amyotrophic lateral sclerosis in mice and correlate with disease progression in patients with amyotrophic lateral sclerosis. Brain 2011, 134: 1293–1314.
Reynolds AD, Banerjee R, Liu J, Gendelman HE, Mosley RL. Neuroprotective activities of CD4+CD25+ regulatory T cells in an animal model of Parkinson’s disease. J Leukoc Biol 2007, 82: 1083–1094.
Leiter O, Kempermann G, Walker TL. A common language: how neuroimmunological cross talk regulates adult hippocampal neurogenesis. Stem Cells Int 2016, 2016: 1681590.
Trzonkowski P, Szmit E, Mysliwska J, Mysliwski A. CD4+CD25+ T regulatory cells inhibit cytotoxic activity of CTL and NK cells in humans-impact of immunosenescence. Clin Immunol 2006, 119: 307–316.
Ye M, Chung HS, Lee C, Yoon MS, Yu AR, Kim JS, et al. Neuroprotective effects of bee venom phospholipase A2 in the 3xTg AD mouse model of Alzheimer’s disease. J Neuroinflammation 2016, 13: 10.
Appel SH, Beers DR, Henkel JS. T cell-microglial dialogue in Parkinson’s disease and amyotrophic lateral sclerosis: are we listening? Trends Immunol 2010, 31: 7–17.
Kovaiou RD, Grubeck-Loebenstein B. Age-associated changes within CD4+ T cells. Immunol Lett 2006, 107: 8–14.
Chen Z, Lin F, Gao Y, Li Z, Zhang J, Xing Y, et al. FOXP3 and RORgammat: transcriptional regulation of Treg and Th17. Int Immunopharmacol 2011, 11: 536–542.
Stone DK, Reynolds AD, Mosley RL, Gendelman HE. Innate and adaptive immunity for the pathobiology of Parkinson’s disease. Antioxid Redox Signal 2009, 11: 2151–2166.
Deleidi M, Gasser T. The role of inflammation in sporadic and familial Parkinson’s disease. Cell Mol Life Sci 2013, 70: 4259–4273.
Monahan AJ, Warren M, Carvey PM. Neuroinflammation and peripheral immune infiltration in Parkinson’s disease: an autoimmune hypothesis. Cell Transplant 2008, 17: 363–372.
Brochard V, Combadiere B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, et al. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest 2009, 119: 182–192.
Saunders JA, Estes KA, Kosloski LM, Allen HE, Dempsey KM, Torres-Russotto DR, et al. CD4+ regulatory and effector/memory T cell subsets profile motor dysfunction in Parkinson’s disease. J Neuroimmune Pharmacol 2012, 7: 927–938.
Stevens CH, Rowe D, Morel-Kopp MC, Orr C, Russell T, Ranola M, et al. Reduced T helper and B lymphocytes in Parkinson’s disease. J Neuroimmunol 2012, 252: 95–99.
Di Santo JP. Natural killer cell developmental pathways: a question of balance. Annu Rev Immunol 2006, 24: 257–286.
Mihara T, Nakashima M, Kuroiwa A, Akitake Y, Ono K, Hosokawa M, et al. Natural killer cells of Parkinson’s disease patients are set up for activation: a possible role for innate immunity in the pathogenesis of this disease. Parkinsonism Relat Disord 2008, 14: 46–51.
Niwa F, Kuriyama N, Nakagawa M, Imanishi J. Effects of peripheral lymphocyte subpopulations and the clinical correlation with Parkinson’s disease. Geriatr Gerontol Int 2012, 12: 102–107.
Baldereschi M, Di Carlo A, Rocca WA, Vanni P, Maggi S, Perissinotto E, et al. Parkinson’s disease and parkinsonism in a longitudinal study: two-fold higher incidence in men. ILSA Working Group. Italian Longitudinal Study on Aging. Neurology 2000, 55: 1358–1363.
Taylor KS, Cook JA, Counsell CE. Heterogeneity in male to female risk for Parkinson’s disease. J Neurol Neurosurg Psychiatry 2007, 78: 905–906.
Elbaz A, Bower JH, Maraganore DM, McDonnell SK, Peterson BJ, Ahlskog JE, et al. Risk tables for parkinsonism and Parkinson’s disease. J Clin Epidemiol 2002, 55: 25–31.
Gillies GE, Pienaar IS, Vohra S, Qamhawi Z. Sex differences in Parkinson’s disease. Front Neuroendocrinol 2014, 35: 370–384.
Pavon JM, Whitson HE, Okun MS. Parkinson’s disease in women: a call for improved clinical studies and for comparative effectiveness research. Maturitas 2010, 65: 352–358.
Ragonese P, D’Amelio M, Salemi G, Aridon P, Gammino M, Epifanio A, et al. Risk of Parkinson disease in women: effect of reproductive characteristics. Neurology 2004, 62: 2010–2014.
Popat RA, Van Den Eeden SK, Tanner CM, McGuire V, Bernstein AL, Bloch DA, et al. Effect of reproductive factors and postmenopausal hormone use on the risk of Parkinson disease. Neurology 2005, 65: 383–390.
Cunningham M, Gilkeson G. Estrogen receptors in immunity and autoimmunity. Clin Rev Allergy Immunol 2011, 40: 66–73.
Karpuzoglu E, Zouali M. The multi-faceted influences of estrogen on lymphocytes: toward novel immuno-interventions strategies for autoimmunity management. Clin Rev Allergy Immunol 2011, 40: 16–26.
Pernis AB. Estrogen and CD4+ T cells. Curr Opin Rheumatol 2007, 19: 414–420.
Aristimuno C, Teijeiro R, Valor L, Alonso B, Tejera-Alhambra M, de Andres C, et al. Sex-hormone receptors pattern on regulatory T-cells: clinical implications for multiple sclerosis. Clin Exp Med 2012, 12: 247–255.
Ortona E, Pierdominici M, Berstein L. Autoantibodies to estrogen receptors and their involvement in autoimmune diseases and cancer. J Steroid Biochem Mol Biol 2014, 144 Pt B: 260–267.
Acknowledgements
This work was supported by the National Key R&D Program of China (2016YFC1306600), the National Natural Science Foundation of China (81471292, U1603281, U1503222, 81430021), the Science Foundation of Guangdong Province, China (2015A030311021), a Technology Project of Guangzhou Municipality, China (201504281820463), and a Science and Technology Planning Project of Guangdong Province, China (2016A050502025).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that no conflicts of interest exist.
Rights and permissions
About this article
Cite this article
Cen, L., Yang, C., Huang, S. et al. Peripheral Lymphocyte Subsets as a Marker of Parkinson’s Disease in a Chinese Population. Neurosci. Bull. 33, 493–500 (2017). https://doi.org/10.1007/s12264-017-0163-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-017-0163-9