Abstract
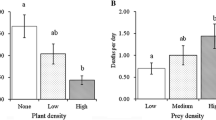
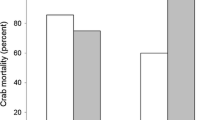
Predation is among the most important biotic factors affecting benthic populations. Habitat complexity, such as seagrass shoot density, can significantly reduce rates of predation by changing predator and prey behaviors, increasing searching and handling times, and reducing encounter rates; this relationship is assumed to be nonlinear. For bay scallops, and other commercially important seagrass-associated prey, understanding the relationship between survival and habitat can have important implications. In this study, we looked at the shape of the habitat survival function (HSF) for bay scallops across four different decapod predator species (Callinectes sapidus, Carcinus maenas, Dyspanopeus sayi, and Libinia sp.) using a series of mesocosm experiments at four different levels of habitat complexity (0, 200, 400, and 800 shoots m−2). As expected, scallop survival was higher in the complex seagrass habitat than when no seagrass was present. However, the shape of the HSF varied among predators: when green crabs were predators, the HSF was linear, whereas the HSF was hyperbolic in the presence of both mud and blue crabs. These data suggest that even small increases of seagrass shoot density from very low levels may rapidly increase prey survival, but that prey survival is unlikely to increase across broad changes in habitat complexity. Further, this experiment suggests that predator identity may be important in determining the relationship between prey survival and habitat complexity. For scallop restoration, efforts can be enhanced by selecting even relatively low levels of seagrass habitat, regardless of perceived “value” based on shoot density.



Similar content being viewed by others
References
Arnold, W.S., N. Blake, M.H. Harrison, D.C. Marelli, M. Parker, S. Peters, and D. Sweat. 2005. Restoration of bay scallop (Argopecten irradians (Lamarck)) populations in Florida coastal waters: planting techniques and the growth, mortality and reproductive development of planted scallops. Journal of Shellfish Research 24: 883–904.
Bartholomew, A. 2002. Total cover and cover quality: predicted and actual effects on a predator’s foraging success. Marine Ecology Progress Series 227: 1–9.
Bartholomew, A., R. Diaz, and G. Cicchetti. 2000. New dimensionless indices of structural habitat complexity: predicted and actual effects on a predator’s foraging success. Marine Ecology Progress Series 206: 45–58.
Bauer, S.I. 1994. The dynamics of mud crab, Dyspanopeus sayi, predation on juvenile bay scallops, Argopecten irradians irradians in eelgrass. MS, State University of New York at Stony Brook.
Belding, D. 1910. A report upon the scallop fishery of Massachusetts. The Commonwealth of Massachusetts, Boston.
Bell, J., and M. Westoby. 1986. The abundance of macrofauna in seagrass is due to habitat preference, not predation. Oecologia 68: 205–209.
Bell, S., R. Brooks, B. Robbins, M. Fonseca, and M. Hall. 2001. Faunal response to fragmentation in seagrass habitats: implications for seagrass conservation. Biological Conservation 1000: 115–123.
Beukers, J.S., and G.P. Jones. 1997. Habitat complexity modifies the impact of piscivores on a coral reef fish population. Oecologia 114: 50–59.
Bishop, M., J. Rivera, E.A. Irlandi, W.G. Ambrose Jr., and C.H. Peterson. 2005. Spatio-temporal patterns in the mortality of bay scallop recruits in North Carolina: investigation of a life history anomaly. Journal of Experimental Marine Biology and Ecology 315: 127–146.
Bologna, P., and K.J. Heck. 1999. Differential predation and growth rates of bay scallops within a seagrass habitat. Journal of Experimental Marine Biology and Ecology 239: 299–314.
Canion, C., and K.L.J. Heck. 2009. Effect of habitat complexity on predation success: re-evaluating the current paradigm in seagrass beds. Marine Ecology Progress Series 393: 37–46.
Carroll, J.M. 2012. The effects of habitat and predation on bay scallop populations in New York. Stony Brook, NY: PhD. Stony Brook University.
Carroll, J., B. Furman, S. Tettelbach, and B. Peterson. 2012. Balancing the edge effects budget: bay scallop settlement and loss along a seagrass edge. Ecology 93: 1637–1647.
Carroll, J., and B. Peterson. 2013. Ecological trade-offs in seascape ecology: bay scallop survival and growth across a seagrass seascape. Landscape Ecology 28: 1401–1413.
Carroll, J., B.J. Peterson, D. Bonal, A. Weinstock, C.F. Smith, and S.T. Tettelbach. 2010. Comparative survival of bay scallops in eelgrass and the introduced alga, Codium fragile, in a New York estuary. Marine Biology 157: 249–259.
Connell, J. 1975. Some mechanisms producing the structure in natural communities: a model and evidence from field experiments. In Ecology and evolution of communities, ed. M. Cody and J. Diamond. Cambridge: Belknap Press of Harvard University Press.
Crowder, L., and W. Cooper. 1982. Habitat structural complexity and interaction between bluegills and their prey. Ecology 63: 1802–1813.
Dorenbosch, M., M.G.G. Grol, A. de Groene, G. van der Velde, and I. Nagelkerken. 2009. Piscivore assemblages and predation pressure affect relative safety of some back-reef habitats for juvenile fish in a Caribbean bay. Marine Ecology Progress Series 379: 181–196.
Eggleston, D., R. Lipcius, and A. Hines. 1992. Density-dependent predation by blue crabs upon infaunal clam species with contrasting distribution and abundance patterns. Marine Ecology Progress Series 85: 55–68.
Eckman, J. 1987. The role of hydrodynamics in recruitment, growth and survival of Argopecten irradians (L.) and Anomia simplex (D’Orbigny) within eelgrass meadows. Journal of Experimental Marine Biology and Ecology 106: 165–191.
Florido, R., and A.J. Sanchez. 2010. Effect of seagrass complexity, prey mobility, and prey density on predation by the blue crab, Callinectes sapidus, (Decapoda, Brachyura). Crustaceana 83: 1069–1089.
Floyd, T., and J. Williams. 2004. Impact of green crab (Carcinus maenas L.) predation on a population of softshell clams (Mya arenaria L.) in the southern Gulf of St. Lawrence. Journal of Shellfish Research 23: 457–462.
Ford, J., R. Williams, A. Fowler, D. Cox, and I. Suthers. 2010. Identifying critical estuarine seagrass habitat for settlement of coastally spawned fish. Marine Ecology Progress Series 408: 181–193.
Garcia-Esquivel, Z., and V.M. Bricelj. 1993. Ontogenetic changes in microhabitat distribution of juvenile bay scallops, Argopecten irradians irradians (L.), in eelgrass beds, and their potential significance to early recruitment. Biological Bulletin 185: 42–55.
Goldberg, R., J. Pereira, and P. Clark. 2000. Strategies for enhancement of natural bay scallop, Argopecten irradians irradians, populations; A case study in the Niantic River estuary, Connecticut, USA. Aquaculture International 8: 139–158.
Gotceitas, V., and P. Colgan. 1989. Predator foraging success and habitat complexity: quantitative test of the threshold hypothesis. Oecologia 80: 158–166.
Grabowski, J. 2004. Habitat complexity disrupts predator-prey interactions but not the trophic cascade on oyster reefs. Ecology 85: 995–1004.
Grabowski, J., A. Hughes, and D. Kimbro. 2008. Habitat complexity influences cascading effects of multiple predators. Ecology 89: 3413–3422.
Graham, S., J. Davis, L. Deegan, J. Cebrain, J. Hughes, and J. Hauxwell. 1998. Effect of eelgrass (Zostera marina) density on the feeding efficiency of the mummichog (Fundulus heteroclitus). Biological Bulletin 195: 241–243.
Harris, L.A., B. Buckley, S.W. Nixon, and B.T. Allen. 2004. Experimental studies of predation by bluefish Pomatomus saltatrix in varying densities of seagrass and macroalgae. Marine Ecology Progress Series 281: 233–239.
Heck, K., G. Hays, and R. Orth. 2003. Critical evaluation of the nursery role hypothesis for seagrass meadows. Marine Ecology Progress Series 253: 123–136.
Heck, K.J., and R. Orth. 1980. Seagrass habitats: the roles of habitat complexity, competition and predation in structuring associated fish and motile macroinvertebrate assemblages. In Estuarine perspectives, ed. V.S. Kennedy. New York: Academic.
Heck, K.J., R. Orth. 2006. Predation in seagrass beds. In: Larkum A, Orth R, Duarte C (eds) Seagrasses: biology, ecology and conservation. Springer, Dordrecht, Netherlands
Heck, K.L.J., K.W. Able, C.T. Roman, and M.P. Fahay. 1995. Composition, abundance, biomass and production of macrofauna in New England estuaries: comparisons among eelgrass meadows and other nursery habitats. Estuaries 18: 379–389.
Heck, K.L.J., and L. Crowder. 1990. Habitat structure and predator-prey interactions. In Habitat complexity: the physical arrangement of objects in space, ed. S. Bell, E. McCoy, and H. Mushinsky. New York: Chapman & Hall.
Heck K.J., and T. Thoman. 1981. Experiments on predator-prey interactions in vegetated aquatic habitats. Journal of Experimental Marine Biology and Ecology 53: 125–134.
Hernandez Cordero, A.L., and R.D. Seitz. 2014. Structured habitat provides a refuge from blue crab, Callinectes sapidus, predation for the bay scallop, Argopecten irradians concentricus (Say 1822). Journal of Experimental Marine Biology and Ecology 460: 100–108.
Hernandez Cordero, A., R. Seitz, R. Lipcius, C. Bovery, and D. Schulte. 2012. Habitat affects survival of translocated bay scallops, Argopecten irradians concentricus (Say 1822), in lower Chesapeake Bay. Estuaries and Coasts 35: 1340–1345.
Hill, J., and M. Weissburg. 2013. Habitat complexity and predator size mediate interactions between intraguild blue crab predators and mud crab prey in oyster reefs. Marine Ecology Progress Series 488: 209–219.
Hovel, K., and R. Lipcius. 2001. Habitat fragmentation in a seagrass landscape: patch size and complexity control blue crab survival. Ecology 82: 1814–1829.
Hovel, K., and R. Lipcius. 2002. Effects of seagrass habitat fragmentation on juvenile blue crab survival and abundance. Journal of Experimental Marine Biology and Ecology 271: 75–98.
Hovel, K.A., and M.S. Fonseca. 2005. Influence of seagrass landscape structure on the juvenile blue crab habitat-survival function. Marine Ecology Progress Series 300: 179–191.
Hovel, K.A., and R. Wahle. 2010. Effects of habitat patchiness on American lobster movement across a gradient of predation risk and shelter competition. Ecology 91: 1993–2002.
Hunt, H., and R.E. Scheibling. 1997. Role of early post-settlement mortality in recruitment of benthic marine invertebrates. Marine Ecology Progress Series 155: 269–301.
Hurlbert, S. H. 1984. Pseudoreplication and the design of ecological field experiments. Ecology Monographs 54: 187–211.
Irlandi, E.A. 1997. Seagrass patch size and survivorship of an infaunal bivalve. Oikos 78: 511–518.
Irlandi, E.A., W.G. Ambrose Jr., and B.A. Orlando. 1995. Landscape ecology and the marine environment: how spatial configuration of seagrass habitat influences growth and survival of the bay scallop. Oikos 72: 307–313.
Irlandi, E.A., B.A. Orlando, and W.G. Ambrose Jr. 1999. Influence of seagrass habitat patch size on growth and survival of juvenile bay scallops, Argopecten irradians concentricus (Say). Journal of Experimental Marine Biology and Ecology 235: 21–43.
Johnson, D.W. 2006. Predation, habitat complexity, and variation in density-dependent mortality of temperate reef fishes. Ecology 87: 1179–1188.
Kulp, R., V. Politano, H. Lane, S. Lombardi, and K.J. Paynter. 2011. Predation of juvenile Crassostrea virginica by two species of mud crabs found in the Chesapeake Bay. Journal of Shellfish Research 30: 261–266.
Lannin, R., and K.A. Hovel. 2011. Variable prey density modifies the effects of seagrass habitat structure on predator-prey interactions. Marine Ecology Progess Series 442: 59–70.
Lipcius, R., D. Eggleston, D. Miller, and T. Luhrs. 1998. The habitat-survival function for Caribbean spiny lobster: an inverted size effect and non-linearity in mixed algal and seagrass habitats. Marine & Freshwater Research 49: 807–816.
Lipcius, R., and A. Hines. 1986. Variable functional responses of a marine predator in dissimilar homgenous microhabitats. Ecology 67: 1361–1371.
Main, K. 1987. Predator avoidance in seagrass meadows: prey behavior, microhabitat selection, and cryptic coloration. Ecology 68: 170–180.
Mattila, J., K.L. Heck, E. Millstein, E. Miller, C. Gustafsson, S. Williams, and D. Byron. 2008. Increased habitat structure does not always provide increased refuge from predation. Marine Ecology Progress Series 361: 15–20.
Menge, B. 1983. Components of predation intensity in the low zone of the New England rocky intertidal region. Oecologia 58: 141–155.
Milke, L., and V. Kennedy. 2001. Mud crabs (Xanthidae) in Chesapeake Bay: claw characteristics and predation on epifaunal bivalves. Invertebrate Biology 120: 67–77.
Miron, G., D. Audet, T. Landry, and M. Moriyasu. 2005. Predation potential of the invasive green crab (Carcinus maenas) and other common predators on commercial bivalve species found on Prince Edward Island. Journal of Shellfish Research 24: 579–586.
Nelson, WG. 1979. Experimental studies of selective predation on ampibpods: Consequences for amphipod distribution and abundance. Journal of Experimental Marine Biology and Ecology 38: 225–245.
Nelson, W. 1981. Experimental studies of decapod and fish predation on seagrass macrobenthos. Marine Ecology Progress Series 5: 141–149.
Nelson, W.G., and E. Bonsdorff. 1990. Fish predation and habitat complexity: are complexity thresholds real? Journal of Experimental Marine Biology and Ecology 141: 183–194.
NYSDEC (New York State Department of Environmental Conservation) 2014. Annual commerial shellfish landings for New York State, 1946–2013. NYSDEC. New York: East Setauket.
O’Connor, N.E., J.H. Grabowski, L.M. Ladwig, and J.F. Bruno. 2008. Simulated predator extinctions: predator identity affects survival and recruitment of oysters. Ecology 89: 428–438.
Orth, R. 1992. A perspective on plant-animal interactions in seagrasses: physical and biological determinants influencing plant and animal abundance. In Plant-animal interactions in marine benthos, book 46, ed. D.J. SHaJP. Oxford: Clarendon.
Orth, R., and J. van Montfrans. 2002. Habitat quality and prey size as determinants of survival in postlarval and early juvenile instars of the blue crab Callinectes sapidus. Marine Ecology Progress Series 231: 205–213.
Orth, R., K.J. Heck, and J. Van Montfrans. 1984. Faunal communities in seagrass beds: a review of the influence of plant structure and prey characteristics on predator-prey relationships. Estuaries 7: 339–350.
Orth, R., T. Carruthers, W. Dennison, C. Duarte, J. Fourqurean, K.J. Heck, A. Hughes, G. Kendrick, W. Kenworthy, S. Olyarnik, F. Short, M. Waycott, and S. Williams. 2006. A global crisis for seagrass ecosystems. Bioscience 56: 987–996.
Pohle, D.G., V.M. Bricelj, and Z. Garcia-Esquivel. 1991. The eelgrass canopy: an above bottom refuge from benthic predators for juvenile bay scallops Argopecten irradians. Marine Ecology Progress Series 74: 47–59.
Polyakov, O., J.N. Krauter, E.E. Hofmann, S.C. Buckner, V.M. Bricelj, E.N. Powell, and J.M. Klinck. 2007. Benthic predators and nothern quahog (=hard clam) (Mercenaria mercenaria Linnaeus, 1758) populations. Journal of Shellfish Research 26: 995–1010.
Prescott, R.C. 1990. Sources of predatory mortality in the bay scallop Argopecten irradians (Lamark): interactions with seagrass and epibiotic coverage. Journal of Experimental Marine Biology and Ecology 144: 63–83.
R Development Core Team. 2013. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, http://www.R-project.org/.
Ray-Culp, M., M. Davis, and A.W. Stoner. 1999. Predation by xanthid crabs on early post-settlement gastropods: the role of prey size, prey density, and habitat complexity. Journal of Experimental Marine Biology and Ecology 240: 303–321.
Rindone, R., and D. Eggleston. 2011. Predator-prey dynamics between recently established stone crabs (Menippe spp.) and oyster prey (Crassostrea virginica). Journal of Experimental Marine Biology and Ecology 407: 216–255.
Ropes, J. 1989. The food habits of five crab species at Pettaquamscutt River, Rhode Island. Fishery Bulletin 87: 197–204.
Russo, A.R. 1987. Role of habitat complexity in mediating predation by the gray damselfish Abudefduf sordidus on epiphytal amphipods. Marine Ecology Progress Series 36: 101–105.
Scheinin, M., S.B. Scyphers, L. Kauppi, K.L.J. Heck, and J. Mattila. 2012. The relationship between vegetation density and its protective value depends on the densities and traits of prey and predators. Oikos 121: 1093–1102.
Schulman, J. 1996. Habitat complexity as a determinant of juvenile blue crab survival. MSc., The College of William and Mary, Gloucester Point, VA.
Seed, R. 1980. Predator-prey relationships between the mud crab Panopeus herbstii, the blue crab, Callinectes sapidus and the Atlantic ribbed mussel Guekensia (=Modiolus) demissa. Estuarine and Coastal Marine Science 11: 445–458.
Seed, R. 1993. Invertebrate predators and their role in structuring coastal and estuarine populations of filter feeding bivalves. In Bivalve filter feeders, ed. R. Dame. Berlin Heidelberg: Springer.
Seed, R., and R. Hughes. 1995. Criteria for prey-size selection in molluscan crabs with contrasting claw morphologies. Journal of Experimental Marine Biology and Ecology 193: 177–195.
Seitz, R., R. Lipcius, A. Hines, and D. Eggleston. 2001. Density-dependent predation, habitat variation, and the persistence of marine bivalve prey. Ecology 82: 2435–2451.
Stoner, A.W. 2009. Habitat-mediated survival of newly settled red king crab in the presence of a predatory fish: role of habitat complexity and heterogeneity. Journal of Experimental Marine Biology and Ecology 382: 54–60.
Streib, M.D., V.M. Bricelj, and S.I. Bauer. 1995. Population biology of the mud crab, Dyspanopeus sayi, an important predator of juvenile bay scallops in Long Island (USA) eelgrass beds. Journal of Shellfish Research 14: 347–357.
Tettelbach, S. 1986. Dynamics of crustacean predation on the northern bay scallop, Argopecten irradians. University of Connecticut.
Tettelbach, S.T., and C.F. Smith. 2009. Bay scallop restoration in New York. Ecological Restoration 27: 20–22.
Tettelbach, S., B. Peterson, J. Carroll, S. Hughes, D. Bonal, A. Weinstock, J. Europe, B. Furman, and C. Smith. 2013. Priming the larval pump: resurgence of bay scallop populations following initiation of intensive restoration efforts. Marine Ecology Progress Series 478: 153–172.
Thayer, G.W., and H.H. Stuart. 1974. The bay scallop makes its bed of seagrass. Marine Fisheries Review 36: 27–30.
Virnstein, R., and M. Curran. 1986. Colonization of artificial seagrass versus time and distance from source. Marine Ecology Progress Series 29: 279–288.
Warren, M., R. Gregory, B. Laurel, and P. Snelgrove. 2010. Increasing density of juvenile Atlantic (Gadus morhua) and Greenland cod (G. ogac) in association with spatial expansion and recovery of eelgrass (Zostera marina) in a coastal nursery habitat. Journal of Experimental Marine Biology and Ecology 394: 154–160.
Wong, M.C. 2013. Green crab (Carcinus maenas Linnaeus, 1758)) foraging on soft-shell clams (Mya arenaria Linnaeus, 1758) across seagrass complexity: behavioral mechanisms and a new habitat complexity index. Journal of Experimental Marine Biology and Ecology 446: 139–150.
Acknowledgments
We would like to thank J. Voci, P. Miller, A. Stubler, V. D’Ambrosia, R. Kulp, and B. Furman of Stony Brook University for help with field collections of animals and in mesocosm processing. In addition, we would like to thank bayman Frank Sloup, of Crabs Unlimited, Bayshore, Long Island, NY, for donating blue crabs for experiments. We would also like to thank Dr. Stephen Tettelbach of Long Island University, Dr. Robert Cerrato of Stony Brook University, Dr. David Eggleston of NC State, and Drs. Ken Heck and John Valentine of Dauphin Island Sea Lab and two anonymous reviewers for insightful comments. Finally, we would like to thank G. Rivara and M. Patricio of the Cornell Cooperative Extension of Suffolk County’s shellfish hatchery for donating scallops for this project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Lawrence P. Rozas
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 371 kb)
Rights and permissions
About this article
Cite this article
Carroll, J.M., Jackson, L.J. & Peterson, B.J. The Effect of Increasing Habitat Complexity on Bay Scallop Survival in the Presence of Different Decapod Crustacean Predators. Estuaries and Coasts 38, 1569–1579 (2015). https://doi.org/10.1007/s12237-014-9902-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-014-9902-6