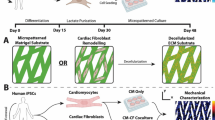
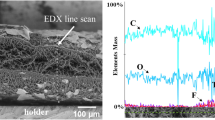
Abstract
Despite recent advances in biomimetic substrates, there is still only limited understanding of how the extracellular matrix (ECM) functions in the maintenance of cardiomyocyte (CM) phenotype. In this study, we designed electrospun substrates inspired by morphologic features of non-failing and failing human heart ECM, and examined how these substrates regulate phenotypes of adult and neonatal rat ventricular CMs (ARVM and NRVM, respectively). We found that poly(ε-caprolactone) fiber substrates designed to mimic the organized ECM of a non-failing human heart maintained healthy CM phenotype (evidenced by cell morphology, organized actin/myomesin bands and expression of β-MYH7 and SCN5A.1 and SCN5A.2) compared to both failing heart ECM-mimetic substrates and tissue culture plates. Moreover, culture of ARVMs and NRVMs on aligned substrates showed differences in m- and z-line alignment; with ARVMs aligning parallel to the ECM fibers and the NRVMs aligning perpendicular to the fibers. The results provide new insight into cardiac tissue engineering by illustrating the importance models that mimic the cardiac ECM microenvironment in vitro.




Similar content being viewed by others
References
Akhyari, P., H. Kamiya, A. Haverich, M. Karck, and A. Lichtenberg. Myocardial tissue engineering: the extracellular matrix. Eur. J. Cardio-Thorac. Surg. 34:229–241, 2008.
Ausma, J., and M. Borgers. Dedifferentiation of atrial cardiomyocytes: from in vivo to in vitro. Cardiovasc. Res. 55:9–12, 2002.
Bhana, B., R. K. Iyer, W. L. Chen, R. Zhao, K. L. Sider, M. Likhitpanichkul, C. A. Simmons, and M. Radisic. Influence of substrate stiffness on the phenotype of heart cells. Biotechnol. Bioeng. 105:1148–1160, 2010.
Bird, S. D., P. A. Doevendans, M. A. van Rooijen, A. de Brutel la Riviere, R. J. Hassink, R. Passier, and C. L. Mummery. The human adult cardiomyocyte phenotype. Cardiovasc. Res. 58:423–434, 2003.
Bugaisky, L. B., and R. Zak. Differentiation of adult rat cardiac myocytes in cell culture. Circ. Res. 64:493–500, 1989.
Bursac, N., M. Papadaki, R. J. Cohen, F. J. Schoen, S. R. Eisenberg, R. Carrier, G. Vunjak-Novakovic, and L. E. Freed. Cardiac muscle tissue engineering: toward an in vitro model for electrophysiological studies. Am. J. Physiol. 277:H433–H444, 1999.
Chlopcikova, S., J. Psotova, and P. Miketova. Neonatal rat cardiomyocytes: a model for the study of morphological, biochemical and electrophysiological characteristics of the heart. Biomed. Pap. Med. Fac. Univ. Palacký Olomouc Czechoslovakia 145:49–55, 2001.
Crapo, P. M., T. W. Gilbert, and S. F. Badylak. An overview of tissue and whole organ decellularization processes. Biomaterials 32:3233–3243, 2011.
Duling, R. R., R. B. Dupaix, N. Katsube, and J. Lannutti. Mechanical characterization of electrospun polycaprolactone (pcl): a potential scaffold for tissue engineering. J. Biomech. Eng. 130:011006–011006, 2008.
Ellingsen, O., A. J. Davidoff, S. K. Prasad, H. J. Berger, J. P. Springhorn, J. D. Marsh, R. A. Kelly, and T. W. Smith. Adult rat ventricular myocytes cultured in defined medium: phenotype and electromechanical function. Am. J. Physiol. 265:H747–H754, 1993.
Engelmayr, Jr., G. C., M. Cheng, C. J. Bettinger, J. T. Borenstein, R. Langer, and L. E. Freed. Accordion-like honeycombs for tissue engineering of cardiac anisotropy. Nat. Mater. 7:1003–1010, 2008.
Farouz, Y., Y. Chen, A. Terzic, and P. Menasche. Concise review: growing hearts in the right place: on the design of biomimetic materials for cardiac stem cell differentiation. Stem Cells 33:1021–1035, 2015.
Galie, P. A., N. Khalid, K. E. Carnahan, M. V. Westfall, and J. P. Stegemann. Substrate stiffness affects sarcomere and costamere structure and electrophysiological function of isolated adult cardiomyocytes. Cardiovasc. Pathol. 22:219–227, 2013.
Galindo, C. L., E. Kasasbeh, A. Murphy, S. Ryzhov, S. Lenihan, F. A. Ahmad, P. Williams, A. Nunnally, J. Adcock, Y. Song, F. E. Harrell, T. L. Tran, T. J. Parry, J. Iaci, A. Ganguly, I. Feoktistov, M. K. Stephenson, A. O. Caggiano, D. B. Sawyer, and J. H. Cleator. Anti-remodeling and anti-fibrotic effects of the neuregulin-1beta glial growth factor 2 in a large animal model of heart failure. J. Am. Heart Assoc. 3:e000773, 2014.
Golden, H. B., D. Gollapudi, F. Gerilechaogetu, J. Li, R. J. Cristales, X. Peng, and D. E. Dostal. Isolation of cardiac myocytes and fibroblasts from neonatal rat pups. Methods Mol. Biol. 843:205–214, 2012.
Gupta, M. K., J. M. Walthall, R. Venkataraman, S. W. Crowder, D. K. Jung, S. S. Yu, T. K. Feaster, X. Wang, T. D. Giorgio, C. C. Hong, F. J. Baudenbacher, A. K. Hatzopoulos, and H.-J. Sung. Combinatorial polymer electrospun matrices promote physiologically-relevant cardiomyogenic stem cell differentiation. PLoS One 6:e28935, 2011.
Hang, C. T., J. Yang, P. Han, H. L. Cheng, C. Shang, E. Ashley, B. Zhou, and C. P. Chang. Chromatin regulation by Brg1 underlies heart muscle development and disease. Nature 466:62–67, 2010.
Herron, T. J., F. S. Korte, and K. S. McDonald. Loaded shortening and power output in cardiac myocytes are dependent on myosin heavy chain isoform expression. Am. J. Physiol. Heart Circ. Physiol. 281(3):H1217–H1222, 2001.
Huang, Y., L. Zheng, X. Gong, X. Jia, W. Song, M. Liu, and Y. Fan. Effect of cyclic strain on cardiomyogenic differentiation of rat bone marrow derived mesenchymal stem cells. PLoS One 7:e34960, 2012.
Huyghe, J. M., D. H. van Campen, T. Arts, and R. M. Heethaar. The constitutive behaviour of passive heart muscle tissue: a quasi-linear viscoelastic formulation. J. Biomech. 24:841–849, 1991.
Inserte, J., V. Hernando, M. Ruiz-Meana, M. Poncelas-Nozal, C. Fernandez, L. Agullo, C. Sartorio, U. Vilardosa, and D. Garcia-Dorado. Delayed phospholamban phosphorylation in post-conditioned heart favours ca2+ normalization and contributes to protection. Cardiovasc. Res. 103:542–553, 2014.
Kabaeva, Z., M. Zhao, and D. E. Michele. Blebbistatin extends culture life of adult mouse cardiac myocytes and allows efficient and stable transgene expression. Am. J. Physiol. Heart Circ. Physiol. 294:H1667–H1674, 2008.
Kim, D. H., E. A. Lipke, P. Kim, R. Cheong, S. Thompson, M. Delannoy, K. Y. Suh, L. Tung, and A. Levchenko. Nanoscale cues regulate the structure and function of macroscopic cardiac tissue constructs. Proc. Natl. Acad. Sci. USA 107:565–570, 2010.
Knöll, R. A role for membrane shape and information processing in cardiac physiology. Pflug. Arch. 467:167–173, 2015.
Kovács, M., J. Tóth, C. Hetényi, A. Málnási-Csizmadia, and J. R. Sellers. Mechanism of blebbistatin inhibition of myosin II. J. Biol. Chem. 279:35557–35563, 2004.
Kubin, T., J. Pöling, S. Kostin, P. Gajawada, S. Hein, W. Rees, A. Wietelmann, M. Tanaka, H. Lörchner, S. Schimanski, M. Szibor, H. Warnecke, and T. Braun. Oncostatin m is a major mediator of cardiomyocyte dedifferentiation and remodeling. Cell Stem Cell 9:420–432, 2011.
Kuo, P.-L., H. Lee, M.-A. Bray, N. A. Geisse, Y.-T. Huang, W. J. Adams, S. P. Sheehy, and K. K. Parker. Myocyte shape regulates lateral registry of sarcomeres and contractility. Am. J. Pathol. 181:2030–2037, 2012.
Lakshmanan, R., U. M. Krishnan, and S. Sethuraman. Living cardiac patch: the elixir for cardiac regeneration. Expert Opin. Biol. Ther. 12:1623–1640, 2012.
Louch, W. E., K. A. Sheehan, and B. M. Wolska. Methods in cardiomyocyte isolation, culture, and gene transfer. J. Mol. Cell. Cardiol. 51:288–298, 2011.
Lutolf, M. P., and J. A. Hubbell. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 23:47–55, 2005.
McCain, M. L., and K. K. Parker. Mechanotransduction: the role of mechanical stress, myocyte shape, and cytoskeletal architecture on cardiac function. Pflug. Arch. 462:89–104, 2011.
Mitcheson, J. S., J. C. Hancox, and A. J. Levi. Action potentials, ion channel currents and transverse tubule density in adult rabbit ventricular myocytes maintained for 6 days in cell culture. Pflug. Arch. 431:814–827, 1996.
Modis, L. Organization of the extracellular matrix. Taylor & Francis: CRC Press, 1990.
Morrisey, E. E. Rewind to recover: dedifferentiation after cardiac injury. Cell Stem Cell 9:387–388, 2011.
Norris, R. A., T. K. Borg, J. T. Butcher, T. A. Baudino, I. Banerjee, and R. R. Markwald. Neonatal and adult cardiovascular pathophysiological remodeling and repair. Ann. N. Y. Acad. Sci. 1123:30–40, 2008.
Parameswaran, S., S. Kumar, R. S. Verma, and R. K. Sharma. Cardiomyocyte culture: an update on the in vitro cardiovascular model and future challenges. Can. J. Physiol. Pharmacol. 91:985–998, 2013.
Patel, A., B. Fine, M. Sandig, and K. Mequanint. Elastin biosynthesis: the missing link in tissue-engineered blood vessels. Cardiovasc. Res. 71:40–49, 2006.
Sander, V., G. Suñe, C. Jopling, C. Morera, and J. C. I. Belmonte. Isolation and in vitro culture of primary cardiomyocytes from adult zebrafish hearts. Nat. Protoc. 8:800–809, 2013.
Shutova, M., C. Yang, J. M. Vasiliev, and T. Svitkina. Functions of nonmuscle myosin II in assembly of the cellular contractile system. PLoS One 7:e40814, 2012.
Simpson, D. G., L. Terracio, M. Terracio, R. L. Price, D. C. Turner, and T. K. Borg. Modulation of cardiac myocyte phenotype in vitro by the composition and orientation of the extracellular matrix. J. Cell. Physiol. 161:89–105, 1994.
Sreejit, P., and R. S. Verma. Natural ECM as biomaterial for scaffold based cardiac regeneration using adult bone marrow derived stem cells. Stem Cell Rev. 9:158–171, 2013.
Stout, D. A., J. Yoo, A. N. Santiago-Miranda, and T. J. Webster. Mechanisms of greater cardiomyocyte functions on conductive nanoengineered composites for cardiovascular application. Int. J. Nanomed. 7:5653–5669, 2012.
Valencik, M. L., D. Zhang, B. Punske, P. Hu, J. A. McDonald, and S. E. Litwin. Integrin activation in the heart: a link between electrical and contractile dysfunction? Circ. Res. 99:1403–1410, 2006.
Wang, N., K. Burugapalli, W. Song, J. Halls, F. Moussy, Y. Zheng, Y. Ma, Z. Wu, and K. Li. Tailored fibro-porous structure of electrospun polyurethane membranes, their size-dependent properties and trans-membrane glucose diffusion. J. Membr. Sci. 427:207–217, 2013.
Wendel, J. S., L. Ye, P. Zhang, R. T. Tranquillo, and J. J. Zhang. Functional consequences of a tissue-engineered myocardial patch for cardiac repair in a rat infarct model. Tissue Eng. Part A. 20:1325–1335, 2014.
Acknowledgments
This study was supported by NIH HL091465, NSF DMR 1006558, U0100398, and AHA 13GRNT16690019. The authors would also like to acknowledge the use of resources at the Vanderbilt Institute of Nanoscale Science and Engineering (VINSE), a facility renovated under NSF ARI-R2 DMR-0963361.
Conflict of interest
Rutwik Rath, Jung Bok Lee, Truc-Linh Tran, Sean F. Lenihan, Cristi L. Galindo, Yan Ru Su, Tarek Absi, Leon M. Bellan, Douglas B. Sawyer, and Hak-Joon Sung declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
All human subject research was carried out in accordance with the guidelines of Vanderbilt IRB and human research protection program and approved by approved by the Vanderbilt Institutional Review Board. All animal studies were carried out in accordance with the guidelines of the Vanderbilt University Animal Care and Use Committee and approved by the Vanderbilt Institutional Review Board.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Associate Editor Tanmay Lele oversaw the review of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rath, R., Lee, J.B., Tran, TL. et al. Biomimetic Microstructure Morphology in Electrospun Fiber Mats is Critical for Maintaining Healthy Cardiomyocyte Phenotype. Cel. Mol. Bioeng. 9, 107–115 (2016). https://doi.org/10.1007/s12195-015-0412-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-015-0412-9