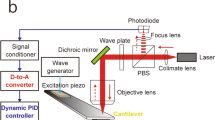
Abstract
Over the past few decades, single molecule investigations employing optical tweezers, AFM and TIRF microscopy have revealed that molecular behaviors are typically characterized by discrete steps or events that follow changes in protein conformation. These events, that manifest as steps or jumps, are short-lived transitions between otherwise more stable molecular states. A major limiting factor in determining the size and timing of the steps is the noise introduced by the measurement system. To address this impediment to the analysis of single molecule behaviors, step detection algorithms incorporate large records of data and provide objective analysis. However, existing algorithms are mostly based on heuristics that are not reliable and lack objectivity. Most of these step detection methods require the user to supply parameters that inform the search for steps. They work well, only when the signal to noise ratio (SNR) is high and stepping speed is low. In this report, we have developed a novel step detection method that performs an objective analysis on the data without input parameters, and based only on the noise statistics. The noise levels and characteristics can be estimated from the data providing reliable results for much smaller SNR and higher stepping speeds. An iterative learning process drives the optimization of step-size distributions for data that has unimodal step-size distribution, and produces extremely low false positive outcomes and high accuracy in finding true steps. Our novel methodology, also uniquely incorporates compensation for the smoothing affects of probe dynamics. A mechanical measurement probe typically takes a finite time to respond to step changes, and when steps occur faster than the probe response time, the sharp step transitions are smoothed out and can obscure the step events. To address probe dynamics we accept a model for the dynamic behavior of the probe and invert it to reveal the steps. No other existing method addresses the impact of probe dynamics on step detection. Importantly, we have also developed a comprehensive set of tools to evaluate various existing step detection techniques. We quantify the performance and limitations of various step detection methods using novel evaluation scales. We show that under these scales, our method provides much better overall performance. The method is validated on different simulated test cases, as well as experimental data.













Similar content being viewed by others
References
Aggarwal, T., and M. Salapaka. Real-time nonlinear correction of back-focal-plane detection in optical tweezers. Rev. Sci. Instrum. 81(12):123105–123105-5, 2010.
Block, S. Kinesin motor mechanics: binding, stepping, tracking, gating, and limping. Biophys. J. 92(9):2986–2995, 2007.
Coppin, C., J. Finer, J. Spudich, and R. Vale. Detection of sub-8-nm movements of kinesin by high-resolution optical-trap microscopy. Proc. Natl. Acad. Sci. USA. 93(5):1913–1917, 1996.
Cornish, P., and T. Ha. A survey of single-molecule techniques in chemical biology. ACS Chem. Biol. 2(1):53–61, 2007.
Egan, J. Signal detection theory and ROC-analysis. New York: Academic Press, 1975.
Engel, A., and D. Müller. Observing single biomolecules at work with the atomic force microscope. Nat. Struct. Mol. Biol. 7(9):715–718, 2000.
Fernandez, J., and H. Li. Force-clamp spectroscopy monitors the folding trajectory of a single protein. Science 303(5664):1674–1678, 2004.
Hancock, W., and J. Howard. Processivity of the motor protein kinesin requires two heads. J. Cell Biol. 140(6):1395–1405, 1998.
Hegner, M., P. Wagner, and G. Semenza. Ultralarge atomically flat template-stripped Au surfaces for scanning probe microscopy. Surf. Sci. 291(1–2):39–46, 1993.
Hirokawa, N., Y. Noda, Y. Tanaka, and S. Niwa. Kinesin superfamily motor proteins and intracellular transport. Nat. Rev. Mol. Cell Biol. 10(10):682–696, 2009.
Hyeon, C., S. Klumpp, and J. Onuchic. Kinesin’s backsteps under mechanical load. Phys. Chem. Chem. Phys. 11(24):4899–4910, 2009.
Kubelka, J., J. Hofrichter, and W. Eaton. The protein folding ‘speed limit’. Curr. Opin. Struct. Biol. 14(1):76–88, 2004.
Lang, M., C. Asbury, J. Shaevitz, and S. Block. An automated two-dimensional optical force clamp for single molecule studies. Biophys. J. 83(1):491–501, 2002.
Materassi, D., P. Baschieri, B. Tiribilli, G. Zuccheri, and B. Samorì. An open source/real-time atomic force microscope architecture to perform customizable force spectroscopy experiments. Rev. Sci. Instrum. 80:084301, 2009.
McGrail, M., J. Gepner, A. Silvanovich, S. Ludmann, M. Serr, and T. Hays. Regulation of cytoplasmic dynein function in vivo by the Drosophila Glued complex. J. Cell Biol. 131(2):411–425, 1995.
Moffitt, J., Y. Chemla, S. Smith, and C. Bustamante. Recent advances in optical tweezers. Biochemistry 77(1):205–228, 2008.
Nishiyama, M., E. Muto, Y. Inoue, T. Yanagida, and H. Higuchi. Substeps within the 8-nm step of the ATPase cycle of single kinesin molecules. Nat. Cell Biol. 3(4):425–428, 2001.
Oberhauser, A., P. Hansma, M. Carrion-Vazquez, and J. Fernandez. Stepwise unfolding of titin under force-clamp atomic force microscopy. Proc. Natl. Acad. Sci. USA. 98(2):468–472, 2001.
Rief, M., R. Rock, A. Mehta, M. Mooseker, R. Cheney, and J. Spudich. Myosin-V stepping kinetics: a molecular model for processivity. Proc. Natl. Acad. Sci. USA. 97(17):9482–9486, 2000.
Sakamoto, T., I. Amitani, E. Yokota, and T. Ando. Direct observation of processive movement by individual myosin V molecules. Biochem. Biophys. Res. Commun. 272(2):586–590, 2000.
Sakmann, B. Elementary steps in synaptic transmission revealed by currents through single ion channels. Biosci. Rep. 12(4):237–262, 1992.
Schneidman, E., B. Freedman, and I. Segev. Ion channel stochasticity may be critical in determining the reliability and precision of spike timing. Neural Comput. 10(7):1679–1703, 1998.
Sehgal, H., T. Aggarwal, and M. Salapaka. High bandwidth force estimation for optical tweezers. Appl. Phys. Lett. 94(15):153114, 2009.
Sniedovich, M. Dynamic Programming. New York: CRC Press, 2009.
Svoboda, K., C. Schmidt, B. Schnapp, and S. Block. Direct observation of kinesin stepping by optical trapping interferometry. Nature 365(6448):721–727, 1993.
Vale, R., T. Reese, and M. Sheetz. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell 42(1):39–50, 1985.
Vershinin, M., B. Carter, D. Razafsky, S. King, and S. Gross. Multiple-motor based transport and its regulation by Tau. Proc. Natl. Acad. Sci. USA. 104(1):87–92, 2007.
Acknowledgments
The authors would like to thank Melissa Gardner for useful discussions. This work was partially supported by a NIH award (RO1GM044757) to T.S. Hays and NSF grants (ECCS 0802117, CMMI 0900113) to M.V. Salapaka.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Alan J. Hunt oversaw the review of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Aggarwal, T., Materassi, D., Davison, R. et al. Detection of Steps in Single Molecule Data. Cel. Mol. Bioeng. 5, 14–31 (2012). https://doi.org/10.1007/s12195-011-0188-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-011-0188-5