Abstract
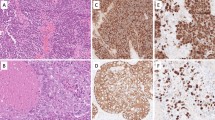
Laryngeal neuroendocrine neoplasms (NENs) are rare and heterogeneous, encompassing well-differentiated neuroendocrine tumors (NETs; grade 1, 2, and 3), neuroendocrine carcinomas (NECs, small cell and large cell types), and mixed neuroendocrine non-neuroendocrine neoplasms (MiNEN). We aimed to study the clinicopathologic spectrum of these neoplasms. A retrospective review of all primary laryngeal NENs diagnosed from 2005 to 2017 was undertaken. Mitotic index was divided into < 2, ≥ 2–10, and > 10 mitoses/2 mm2, with a Ki-67 labelling index of < 2%, ≥ 2–20%, and > 20% for the NET grade 1, 2 and 3 categories, respectively. A total of 27 patients were included. The median age at presentation was 60 years; the male-to-female ratio was 8:1. Supraglottis (n = 22) was the most frequently affected subsite. There were 9 NETs grade 2 (G2), and 18 NECs cases. There were no NET grade 1 or 3 cases in our cohort. Among the NETs G2, the morphology was epithelioid (2), plasmacytoid (3), clear (2), oncocytic (1), and rhabdoid (1). Unique ‘glomeruloid structures’ (n = 5), calcification (n = 3), lymphoid aggregates (n = 5), intranuclear inclusions (n = 2), hyaline globules (n = 3), and Leisegang rings (n = 2) were identified. NECs comprised 16 small cell neuroendocrine carcinoma and 2 large cell neuroendocrine carcinoma. On immunohistochemistry, tumor cells expressed AE1/AE3 (86%), synaptophysin (100%), chromogranin (100%), INSM1 (100%), calcitonin (33.3%). In the NEC group, p53 aberrant expression (87.5%), Retinoblastoma (Rb) loss (88.2%), and diffuse p16 immunoreactivity (66.7%) were additionally observed. Lymph-node metastasis was detected in 62.5% and 85.7%, while distant metastasis in 55.6% and 76.9%, respectively in NET G2 and NEC. Laryngeal NENs are aggressive neoplasms with a high rate of nodal and distant metastasis. Awareness of the wide pathologic spectrum of laryngeal NENs and appropriate use of IHC is needed to render an accurate diagnosis. Ki67 assessment is strongly recommended for laryngeal NEN prognostication



Similar content being viewed by others
References
Goldman NC, Hood CI, Singleton GT. Carcinoid of the larynx. Arch Otolaryngol. 1969;90:64–7.
Wenig BM, Hyams VJ, Heffner DK. Moderately differentiated neuroendocrine carcinoma of the larynx: a clinicopathologic study of 54 cases. Cancer. 1988;62:2658–76.
Ferlito A, Devaney KO, Rinaldo A. Neuroendocrine neoplasms of the larynx: advances in identification, understanding, and management. Oral Oncol. 2006;42:770–88.
Woodruff JM, Huvos AG, Erlandson RA, et al. Neuroendocrine carcinomas of the larynx: a study of two types, one of which mimics thyroid medullary carcinoma. Am J Surg Pathol. 1985;9:771–90.
Shanmugaratnam K. Histological typing of tumours of the upper respiratory tract and ear: World Health Organization International histological classification of tumors. 2nd ed. Berlin: Springer-Verlag; 1991.
Barnes L, Eveson JW, Reichart P, et al. Pathology and genetics of head and neck tumours: World Health Organization classification of tumors. Lyon, France: IARC Press; 2005.
Perez-Ordonez B, Bishop JA, Gnepp DR, et al. Neuroendocrine tumors. In: El-Naggar AK, Chan JKC, Grandis JR, et al., editors. WHO classification of head neck tumours. Lyon: IARC; 2017. p. 95–8.
Strosberg C, Ferlito A, Triantafyllou A, et al. Update on neuroendocrine carcinomas of the larynx. Am J Clin Pathol. 2019;152:686–700.
Rindi G, Klimstra DS, Abedi-Ardekani B, et al. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol. 2018;31(12):1770–86.
Patel SG, Lydiatt WM, Glastonbury CM, et al. Larynx. In: Amin MB, editor., et al., AJCC cancer staging manual. 8th ed. New York, NY: Springer; 2017.
van der Laan TP, Plaat BEC, van der Laan BFAM, Halmos GB. Clinical recommendations on the treatment of neuroendocrine carcinoma of the larynx: a meta-analysis of 436 reported cases. Head Neck. 2015;37:707–15.
Perez-Ordoñez B. Neuroendocrine carcinomas of the larynx and head and neck: challenges in classification and grading. Head Neck Pathol. 2018;12:1–8.
Zhu Y, Gao L, Meng Y, Diao W, Zhu X, Li G, Gao Z, Chen X. Laryngeal neuroendocrine carcinomas: a retrospective study of 14 cases. Biomed Res Int. 2015;2015:832194.
Hunt JL, Ferlito A, Hellquist H, Rinaldo A, Skálová A, Slootweg PJ, et al. Differential diagnosis in neuroendocrine neoplasms of the larynx. Adv Anat Pathol. 2017;24:161–8.
Gavin K, Banville N, Gibbons D, Quinn CM. Liesegang rings in inflammatory breast lesions. J Clin Pathol. 2005;58:1343–4.
Sancheti S, Jain S. Liesegang rings: extremely rare structures in malignant lesions. Int J Surg Pathol. 2018;26:39–40.
Lewis JS, Spence DC, Chiosea S, Barnes EL, Brandwein-Gensler M, El-Mofty SK. Large cell neuroendocrine carcinoma of the larynx: definition of an entity. Head Neck Pathol. 2010;4:198–207.
Kao H-L, Chang W-C, Li W-Y, Chia-Heng Li A, Fen-Yau LA. Head and neck large cell neuroendocrine carcinoma should be separated from atypical carcinoid on the basis of different clinical features, overall survival, and pathogenesis. Am J Surg Pathol. 2012;36:185–92.
Kusafuka K, Abe M, Iida Y, et al. Mucosal large cell neuroendo-crine carcinoma of the head and neck regions in Japanese patients: a distinct clinicopathological entity. J Clin Pathol. 2012;65:704–9.
Feola T, Puliani G, Sesti F, Modica R, Biffoni M, Di Gioia C, Carletti R, Anastasi E, Di Vito V, Centello R, Lenzi A, Isidori AM, Faggiano A, Giannetta E. Laryngeal neuroendocrine tumor with elevated serum calcitonin: a diagnostic and therapeutic challenge. Case report and review of literature. Front Endocrinol. (Lausanne) 2020;11:397
Chou A, Itchins M, de Reuver PR, Arena J, Clarkson A, Sheen A, Sioson L, Cheung V, Perren A, Nahm C, Mittal A, Samra JS, Pajic M, Gill AJ. ATRX loss is an independent predictor of poor survival in pancreatic neuroendocrine tumors. Hum Pathol. 2018;82:249–57.
Alos L, Hakim S, Larque AB, et al. p16 overexpression in high-grade neuroendocrine carcinomas of the head and neck: potential diagnostic pitfall with HPV-related carcinomas. Virchows Arch. 2016;469:277–84.
Bishop JA, Westra WH. Human papillomavirus-related small cell carcinoma of the oropharynx. Am J Surg Pathol. 2011;35:1679–84.
Kraft S, Faquin WC, Krane JF. HPV-associated neuroendo- crine carcinoma of the oropharynx: a rare new entity with potentially aggressive clinical behavior. Am J Surg Pathol. 2012;36:321–30.
Halmos GB, van der Laan TP, van Hemel BM, et al. Is human papillomavirus involved in laryngeal neuroendocrine carci- noma? Eur Arch Otorhinolaryngol. 2013;270:719–25.
Klimstra DS, Klöppel G, La Rosa S, Rindi G. Classification of neuroendocrine neoplasms of the digestive system. In: WHO Classification of Tumours Editorial Board, editors. WHO clas-sification of tumours. Digestive system tumours, 5th ed. Lyon: IARC; 2019. pp. 16–19
Hellquist H, French CA, Bishop JA, Coca-Pelaz A, Propst EJ, PaivaCorreia A, Ngan BY, Grant R, Cipriani NA, Vokes D, Henrique R, Pardal F, Vizcaino JR, Rinaldo A, Ferlito A. NUT midline carcinoma of the larynx: an international series and review of the literature. Histopathology. 2017;70:861–8.
Bahrami A, Gown AM, Baird GS, Hicks MJ, Folpe AL. Aberrant expression of epithelial and neuroendocrine markers in alveolar rhabdomyosarcoma: a potentially serious diagnostic pitfall. Mod Pathol. 2008;21:795–806.
Conlon N, Silva A, Guerra E, Jelinic P, Schlappe BA, Olvera N, Mueller JJ, Tornos C, Jungbluth AA, Young RH, Oliva E, Levine D, Soslow RA. Loss of SMARCA4 expression is both sensitive and specific for the diagnosis of small cell carcinoma of ovary, Hypercalcemic type. Am J Surg Pathol. 2016;40:395–403.
Neves-Silva R, Almeida LY, Silveira HA, Colturato CBN, Duarte A, Ferrisse TM, Silva EV, Vanzolin BF, Bufalino A, Ribeiro-Silva A, León JE. SMARCB1 (INI-1) and NUT immunoexpression in a large series of head and neck carcinomas in a Brazilian reference center. Head Neck. 2020;42:374–84.
Acknowledgements
We sincerely thank Dr Jay Mehta, Center of Oncopathology, Mumbai, for his generous support in IHC standardization, and Shirsat laboratory, ACTREC, Ms. Priti Shenoy and Ms. Neelam for their technical assistance during the study.
Funding
No funding obtained.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by [AS], and [MB]. The first draft of the manuscript was written by [MB] and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in the study were in accordance with the ethical standards of the Institutional Ethics Committee.
Informed Consent
Not required as per institutional ethics committee’s policy for retrospective case series. The authors declare that all information is anonymized and the submission does not include images that may identify any patient.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bal, M., Sharma, A., Rane, S.U. et al. Neuroendocrine Neoplasms of the Larynx: A Clinicopathologic Analysis of 27 Neuroendocrine Tumors and Neuroendocrine Carcinomas. Head and Neck Pathol 16, 375–387 (2022). https://doi.org/10.1007/s12105-021-01367-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-021-01367-9