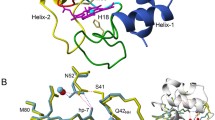
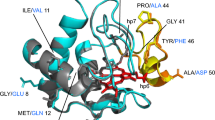
Abstract
Human cytochrome c is a multi-functional protein with key roles in both the mitochondrial electron transfer chain and in apoptosis. In the latter, a complex formed between the mitochondrial phospholipid cardiolipin and cytochrome c is crucial for instigating the release of pro-apoptotic factors, including cytochrome c, from the mitochondrion into the cytosol. The G41S mutant of human cytochrome c is the only known disease-related variant of cytochrome c and causes increased apoptotic activity in patients with autosomal dominant thrombocytopenia. NMR spectroscopy can be used to investigate the interaction of human cytochrome c with cardiolipin and the structural and dynamic factors, which may contribute to enhanced apoptotic activity for the G41S mutant. We present here essentially full backbone amide resonance assignments for ferric human cytochrome c (98 %) as well as assignments of both the ferric (92 %) and ferrous (95 %) forms of the G41S mutant. Backbone amide chemical shift differences between the wild type and G41S mutant in the ferric state reveals significant changes around the mutation site, with many other amides also affected. This suggests the possibility of increased dynamics and/or a change in the paramagnetic susceptibility tensor of the G41S mutant relative to the wild type protein.


Similar content being viewed by others
References
Barker PD et al (2001) A further clue to understanding the mobility of mitochondrial yeast cytochrome c: a (15)N T1rho investigation of the oxidized and reduced species. Eur J Biochem 268:4468–4476
Bradley JM, Silkstone G, Wilson MT, Cheesman MR, Butt JN (2011) Probing a complex of cytochrome C and cardiolipin by magnetic circular dichroism spectroscopy: implications for the initial events in apoptosis. J Am Chem Soc 133:19676–19679
Cai M, Huang Y, Sakaguchi K, Clore GM, Gronenborn AM, Craigie R (1998) An efficient and cost-effective isotope labeling protocol for proteins expressed in Escherichia coli. J Biomol NMR 11:97–102
Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR 6:277–293
Goddard TD, Kneller DG (2008) SPARKY 3 University of California, San Francisco
Hanske J, Toffey JR, Morenz AM, Bonilla AJ, Schiavoni KH, Pletneva EV (2012) Conformational properties of cardiolipin-bound cytochrome c. Proc Natl Acad Sci USA 109:125–130. doi:10.1073/pnas.1112312108
Hong Y, Muenzner J, Grimm SK, Pletneva EV (2012) Origin of the conformational heterogeneity of cardiolipin-bound cytochrome C. J Am Chem Soc 134:18713–18723. doi:10.1021/ja307426k
Huttemann M et al (2011) The multiple functions of cytochrome c and their regulation in life and death decisions of the mammalian cell: from respiration to apoptosis. Mitochondrion 11:369–381. doi:10.1016/j.mito.2011.01.010
Jeng WY, Chen CY, Chang HC, Chuang WJ (2002) Expression and characterization of recombinant human cytochrome c in E. coli. J Bioenerg Biomembr 34:423–431
Josephs TM, Morison IM, Day CL, Wilbanks SM, Ledgerwood EC (2014) Enhancing the peroxidase activity of cytochrome c by mutation of residue 41: implications for the peroxidase mechanism and cytochrome c release. Biochem J 458:259–265. doi:10.1042/bj20131386
Kagan VE et al (2005) Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat Chem Biol 1:223–232
Liu X, Kim CN, Yang J, Jemmerson R, Wang X (1996) Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86:147–157
Moore GR, Pettigrew GW (1990) Cytochrome c: evolutionary, structural and physicochemical aspects. Springer, London
Morison IM et al (2008) A mutation of human cytochrome c enhances the intrinsic apoptotic pathway but causes only thrombocytopenia. Nat Genet 40:387–389
Orekhov VY, Jaravine VA (2011) Analysis of non-uniformly sampled spectra with multi-dimensional decomposition. Prog Nucl Magn Reson Spectrosc 59:271–292. doi:10.1016/j.pnmrs.2011.02.002
Pielak GJ, Auld DS, Betz SF, Hilgen-Willis SE, Garcia LL (1996) Cytochrome c: a multidisciplinary approach. University Science Books, Sausalito
Pollock WB, Rosell FI, Twitchett MB, Dumont ME, Mauk AG (1998) Bacterial expression of a mitochondrial cytochrome c. Trimethylation of lys72 in yeast iso-1-cytochrome c and the alkaline conformational transition. Biochemistry 37:6124–6131
Rajagopal BS, Silkstone GG, Nicholls P, Wilson MT, Worrall JA (2012) An investigation into a cardiolipin acyl chain insertion site in cytochrome c. Biochim Biophys Acta 1817:780–791. doi:10.1016/j.bbabio.2012.02.010
Rajagopal BS et al (2013) The hydrogen-peroxide-induced radical behaviour in human cytochrome c-phospholipid complexes: implications for the enhanced pro-apoptotic activity of the G41S mutant. Biochem J 456:441–452. doi:10.1042/bj20130758
Sinibaldi F, Fiorucci L, Patriarca A, Lauceri R, Ferri T, Coletta M, Santucci R (2008) Insights into cytochrome c-cardiolipin interaction. Role played by ionic strength. Biochemistry 47:6928–6935
Volkov AN, Vanwetswinkel S, Van de Water K, van Nuland NA (2012) Redox-dependent conformational changes in eukaryotic cytochromes revealed by paramagnetic NMR spectroscopy. J Biomol NMR 52:245–256. doi:10.1007/s10858-012-9607-8
Vranken WF et al (2005) The CCPN data model for NMR spectroscopy: development of a software pipeline. Proteins 59:687–696. doi:10.1002/prot.20449
Wang X (2001) The expanding role of mitochondria in apoptosis Genes and development 15:2922–2933
Acknowledgments
We acknowledge the open access use of the Wellcome Trust-funded (grants 083796/Z/07/Z and 099185/Z/12/Z) 900 MHz spectrometer at the HWB-NMR facility at the University of Birmingham and thank Dr Sara Whittaker for assistance with the acquisition of the triple resonance experiments at this facility. This work was supported by a Leverhulme Trust project grant (RPG-2013-164) to JARW and a Leverhulme Trust emeritus fellowship (EM-2014-088) to GRM.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karsisiotis, A.I., Deacon, O.M., Rajagopal, B.S. et al. Backbone resonance assignments of ferric human cytochrome c and the pro-apoptotic G41S mutant in the ferric and ferrous states. Biomol NMR Assign 9, 415–419 (2015). https://doi.org/10.1007/s12104-015-9621-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12104-015-9621-3