Abstract
Objectives
The promoting roles of cyclin dependent kinase 1 (CDK1) have been revealed in various tumors, however, its effects in the progression of cancer stem cells are still confusing. This work aims to explore the roles of CDK1 in regulating the stemness of lung cancer cells.
Methods
Online dataset analysis was performed to evaluate the correlation between CDK1 exression and the survival of lung cancer patients. RT-qPCR, western blot, cell viability, sphere-formation analysis and ALDH activity detection were used to investigate the roles of CDK1 on lung cancer cell stemness, viability and chemotherapeutic sensitivity. Immunocoprecipitation (Co-IP) analysis and rescuing experiments were performed to reveal the underlying mechanisms contributing to CDK1-mediated effects on lung cancer cell stemness.
Results
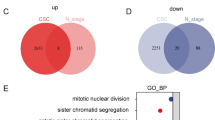
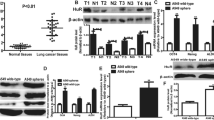
CDK1 mRNA expression was negatively correlated with the overall survival of lung cancer patients and remarkably increased in tumor spheres formed by lung cancer cells compared to the parental cells. Additionally, CDK1 positively regulated the stemness of lung cancer cells. Mechanistically, CDK1 could interact with Sox2 protein, but not other stemness markers (Oct4, Nanog and CD133). Furthermore, CDK1 increased the phosphorylation, cytoplasm-nuclear translocation and transcriptional activity of Sox2 protein in lung cancer cells. Moreover, CDK1 positively regulated the stemness of lung cancer cells in a Sox2-dependent manner. Finally, we revealed that inhibition of CDK1 enhanced the chemotherapeutic sensitivity, which was also rescued by Sox2 overexpression.
Conclusions
This work reveals a novel CDK1/Sox2 axis responsible for maintaining the stemness of lung cancer cells.





Similar content being viewed by others
References
Eide CA, Druker BJ. Understanding cancer from the stem cells up. Nat Med. 2017;23(6):656–7.
Clarke MF. Clinical and therapeutic implications of cancer stem cells. N Engl J Med. 2019;380(23):2237–45.
Buczacki SJA, Popova S, Biggs E, et al. Itraconazole targets cell cycle heterogeneity in colorectal cancer. J Exp Med. 2018;215(7):1891–912.
Zhong L, Hu MM, Bian LJ, Liu Y, Chen Q, Shu HB. Phosphorylation of cGAS by CDK1 impairs self-DNA sensing in mitosis. Cell Discov. 2020;6:26.
Neganova I, Tilgner K, Buskin A, et al. CDK1 plays an important role in the maintenance of pluripotency and genomic stability in human pluripotent stem cells. Cell Death Dis. 2014;5:e1508.
Heo J, Noh BJ, Lee S, et al. Phosphorylation of TFCP2L1 by CDK1 is required for stem cell pluripotency and bladder carcinogenesis. EMBO Mol Med. 2020;12(1):e10880.
Zhang WW, Zhang XJ, Liu HX, et al. Cdk1 is required for the self-renewal of mouse embryonic stem cells. J Cell Biochem. 2011;112(3):942–8.
Ravindran Menon D, Luo Y, Arcaroli JJ, et al. CDK1 Interacts with Sox2 and Promotes Tumor Initiation in Human Melanoma. Cancer Res. 2018;78(23):6561–74.
Menon DR, Fujita M. A state of stochastic cancer stemness through the CDK1-SOX2 axis. Oncotarget. 2019;10(27):2583–5.
Zheng YB, Gong JH, Liu XJ, Li Y, Zhen YS. A CD13-targeting peptide integrated protein inhibits human liver cancer growth by killing cancer stem cells and suppressing angiogenesis. Mol Carcinog. 2017;56(5):1395–404.
Gao L, Sang JZ, Cao H. Limonin enhances the radiosensitivity of nasopharyngeal carcinoma cells via attenuating Stat3-induced cell stemness. Biomed Pharmacother. 2019;118:109366.
Tang Q, Chen Z, Zhao L, Xu H. Circular RNA hsa_circ_0000515 acts as a miR-326 sponge to promote cervical cancer progression through up-regulation of ELK1. Aging (Albany NY). 2019;11(22):9982–99.
Li M, He F, Zhang Z, Xiang Z, Hu D. CDK1 serves as a potential prognostic biomarker and target for lung cancer. J Int Med Res. 2020;48(2):300060519897508.
Gyorffy B, Surowiak P, Budczies J, Lanczky A. Online survival analysis software to assess the prognostic value of biomarkers using transcriptomic data in non-small-cell lung cancer. PLoS ONE. 2013;8(12):e82241.
Zheng L, Li X, Gu Y, Lv X, Xi T. The 3’UTR of the pseudogene CYP4Z2P promotes tumor angiogenesis in breast cancer by acting as a ceRNA for CYP4Z1. Breast Cancer Res Treat. 2015;150(1):105–18.
Qian S, Wang W, Li M. Transcriptional factor Yin Yang 1 facilitates the stemness of ovarian cancer via suppressing miR-99a activity through enhancing its deacetylation level. Biomed Pharmacother. 2020;126:110085.
Shibata M, Hoque MO. Targeting cancer stem cells: a strategy for effective eradication of cancer. Cancers (Basel). 2019;11(5):732.
Yang Y, Li X, Wang T, Guo Q, Xi T, Zheng L. Emerging agents that target signaling pathways in cancer stem cells. J Hematol Oncol. 2020;13(1):60.
Tumpel S, Rudolph KL. Quiescence: good and bad of stem cell aging. Trends Cell Biol. 2019. https://doi.org/10.1016/j.tcb.2019.05.002.
Kobayashi Y, Tanaka T, Mulati M, et al. Cyclin-dependent kinase 1 is essential for muscle regeneration and overload muscle fiber hypertrophy. Front Cell Dev Biol. 2020;8:564581.
Schmidt AK, Pudelko K, Boekenkamp JE, Berger K, Kschischo M, Bastians H. The p53/p73 - p21(CIP1) tumor suppressor axis guards against chromosomal instability by restraining CDK1 in human cancer cells. Oncogene. 2020. https://doi.org/10.1038/s41388-020-01524-4.
Gutierrez A, Kim JO, Umbreit NT, et al. Cdk1 phosphorylation of the Dam1 complex strengthens kinetochore-microtubule attachments. Curr Biol. 2020;30(22):4491-4499.e4495.
Wu G, Lin Q, Lim TK, et al. The interactome of Singapore grouper iridovirus protein ICP18 as revealed by proximity-dependent BioID approach. Virus Res. 2020;291:198218.
Rosendo-Pineda MJ, Vicente JJ, Vivas O, et al. Phosphorylation of NMDA receptors by cyclin B/CDK1 modulates calcium dynamics and mitosis. Commun Biol. 2020;3(1):665.
Li L, Wang J, Hou J, et al. Cdk1 interplays with Oct4 to repress differentiation of embryonic stem cells into trophectoderm. FEBS Lett. 2012;586(23):4100–7.
Zhu Y, Li K, Zhang J, Wang L, Sheng L, Yan L. Inhibition of CDK1 reverses the resistance of 5-fu in colorectal cancer. Cancer Manag Res. 2020;12:11271–83.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Huang, Z., Shen, G. & Gao, J. CDK1 promotes the stemness of lung cancer cells through interacting with Sox2. Clin Transl Oncol 23, 1743–1751 (2021). https://doi.org/10.1007/s12094-021-02575-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-021-02575-z