Abstract
Aims
Pathological response has been shown to be a predictor for survival after preoperative chemotherapy and surgical resection of colorectal cancer liver metastases. This retrospective analysis evaluated the effect on pathological response of adding bevacizumab to standard neoadjuvant chemotherapy in patients with metastatic colorectal cancer (mCRC) and liver metastases.
Methods
Patient records from two Spanish centres were retrospectively examined for this analysis. Patients were included if they had stage IV mCRC with liver metastases, were unresectable or marginally resectable tumour before chemotherapy, and had oxaliplatin- or irinotecan-based chemotherapy, with or without bevacizumab, before resection. Tumour response was evaluated using response evaluation criteria in solid tumours (RECIST). Pathological response was assessed by pathologists blinded to treatment.
Results
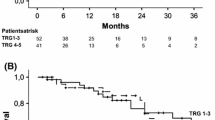
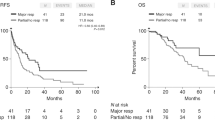
Ninety-five patients were included. Good pathological responses (PR0/PR1) were observed in 37 patients (39 %). The RECIST response rate was 51 %. Only 42 % of patients with a good pathological response had a complete or partial response according to RECIST, while 57 % of those with a poor pathological response had a complete or partial response according to RECIST. RECIST response rates were similar with and without bevacizumab, although 49 % of bevacizumab-treated patients had a good pathological response versus 27 % of those receiving chemotherapy alone (χ 2 P = 0.0302).
Conclusion
Pathological response may be a better indicator of treatment efficacy than RECIST for patients with mCRC receiving bevacizumab in the neoadjuvant setting. Adding bevacizumab to chemotherapy has the potential to increase pathological response rates. Well-designed prospective clinical studies are required to establish the efficacy and tolerability of this approach.


Similar content being viewed by others
References
Faivre J, Manfredi S, Bouvier AM. Epidemiology of colorectal cancer liver metastases. Bull Acad Natl Med. 2003;187:815–22.
Kemeny N. Management of liver metastases from colorectal cancer. Oncology (Williston Park). 2006;20:1161–76.
Adam R, Delvart V, Pascal G, Valeanu A, Castaing D, Azoulay D, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg. 2004;240:644–57.
Cummings LC, Payes JD, Cooper GS. Survival after hepatic resection in metastatic colorectal cancer: a population-based study. Cancer. 2007;109:718–26.
Dexiang Z, Li R, Ye W, Haifu W, Yunshi Z, Qinghai Y, et al. Outcome of patients with colorectal liver metastasis: analysis of 1,613 consecutive cases. Ann Surg Oncol. 2012;19:2860–8.
Lam VW, Spiro C, Laurence JM, Johnston E, Hollands MJ, Pleass HC, et al. A systematic review of clinical response and survival outcomes of downsizing systemic chemotherapy and rescue liver surgery in patients with initially unresectable colorectal liver metastases. Ann Surg Oncol. 2012;19:1292–301.
Dhir M, Lyden ER, Wang A, Smith LM, Ullrich F, Are C. Influence of margins on overall survival after hepatic resection for colorectal metastasis: a meta-analysis. Ann Surg. 2011;254:234–42.
House MG, Ito H, Gönen M, Fong Y, Allen PJ, DeMatteo RP, et al. Survival after hepatic resection for metastatic colorectal cancer: trends in outcomes for 1,600 patients during two decades at a single institution. J Am Coll Surg. 2010;210:744–52.
Wang CC, Li J. An update on chemotherapy of colorectal liver metastases. World J Gastroenterol. 2012;18:25–33.
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42.
Giantonio BJ, Catalano PJ, Meropol NJ, O’Dwyer PJ, Mitchell EP, Alberts SR, et al. Eastern Cooperative Oncology Group Study E3200. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–44.
Ribero D, Wang H, Donadon M, Zorzi D, Thomas MB, Eng C, et al. Bevacizumab improves pathologic response and protects against hepatic injury in patients treated with oxaliplatin-based chemotherapy for colorectal liver metastases. Cancer. 2007;110:2761–7.
Mahfud M, Breitenstein S, El-Badry AS, Puhan M, Rickenbacher A, Samaras P, et al. Impact of preoperative bevacizumab on complications after resection of colorectal liver metastases: case-matched control study. World J Surg. 2010;34:92–100.
Klinger M, Tamandl D, Eipeldauer S, Hacker S, Herberger B, Kaczirek K, et al. Bevacizumab improves pathologic response of colorectal cancer liver metastases treated with XELOX/FOLFOX. Ann Surg Oncol. 2010;17:2059–65.
Wong R, Cunningham D, Barbachano Y, Saffery C, Valle J, Hickish T, et al. A multicentre study of capecitabine, oxaliplatin plus bevacizumab as perioperative treatment of patients with poor-risk colorectal liver-only metastases not selected for upfront resection. Ann Oncol. 2011;22:2042–8.
Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013–9.
Bennouna J, Sastre J, Arnold D, Osterlund P, Greil R, Van Cutsem E, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol. 2013;14:29–37.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Boonsirikamchai P, Asran MA, Maru DM, Vauthey JN, Kaur H, Kopetz S, et al. CT findings of response and recurrence, independent of change in tumor size, in colorectal liver metastasis treated with bevacizumab. AJR Am J Roentgenol. 2011;197:W1060–6.
Vera Garcia R, Gómez Dorronsoro M. Pathological response with bevacizumab in colorectal liver metastases: oncological and pathological review. Cancer Chemother. 2012;7:173–8.
Adam R, Wicherts DA, de Haas RJ, Aloia T, Lévi F, Paule B, et al. Complete pathologic response after preoperative chemotherapy for colorectal liver metastases: myth or reality? J Clin Oncol. 2008;26:1635–41.
Zorcolo L, Rosman AS, Restivo A, Pisano M, Nigri GR, Fancellu A, et al. Complete pathologic response after combined modality treatment for rectal cancer and long-term survival: a meta-analysis. Ann Surg Oncol. 2012;19:2822–32.
Meredith KL, Weber JM, Turaga KK, Siegel EM, McLoughlin J, Hoffe S, et al. Pathologic response after neoadjuvant therapy is the major determinant of survival in patients with esophageal cancer. Ann Surg Oncol. 2010;17:1159–67.
Gruenberger T, Arnold D, Rubbia-Brandt L. Pathologic response to bevacizumab-containing chemotherapy in patients with colorectal liver metastases and its correlation with survival. Surg Oncol. 2012;21:309–15.
Shindoh J, Loyer EM, Kopetz S, Boonsirikamchai P, Maru DM, Chun YS, et al. Optimal morphologic response to preoperative chemotherapy: an alternate outcome end point before resection of hepatic colorectal metastases. J Clin Oncol. 2012;30:4566–72.
Chan G, Hassanain M, Chaudhury P, Vrochides D, Neville A, Cesari M, et al. Pathological response grade of colorectal liver metastases treated with neoadjuvant chemotherapy. HBP (Oxford). 2010;12:277–84.
Rubbia-Brandt L, Giostra E, Brezault C, Roth AD, Andres A, Audard V, et al. Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann Oncol. 2007;18:299–304.
Blazer DG 3rd, Kishi Y, Maru DM, Kopetz S, Chun YS, Overman MJ, et al. Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol. 2008;26:5344–51.
Poultsides GA, Bao F, Servais EL, Hernandez-Boussard T, DeMatteo RP, Allen PJ, et al. Pathologic response to preoperative chemotherapy in colorectal liver metastases: fibrosis, not necrosis, predicts outcome. Ann Surg Oncol. 2012;19:2797–804.
Klinger M, Eipeldauer S, Hacker S, Herberger B, Tamandl D, Dorfmeister M, et al. Bevacizumab protects against sinusoidal obstruction syndrome and does not increase response rate in neoadjuvant XELOX/FOLFOX therapy of colorectal cancer liver metastases. Eur J Surg Oncol. 2009;35:515–20.
Egger ME, Cannon RM, Metzger TL, Nowacki M, Kelly L, Tatum C, et al. Assessment of chemotherapy response in colorectal liver metastases in patients undergoing hepatic resection and the correlation to pathologic residual viable tumor. J Am Coll Surg. 2013;216:845–57.
Ince WL, Jubb AM, Holden SN, Holmgren EB, Tobin P, Sridhar M, et al. Association of k-ras, b-raf, and p53 status with the treatment effect of bevacizumab. J Natl Cancer Inst. 2005;97:981–9.
Hurwitz HI, Yi J, Ince W, Novotny WF, Rosen O. The clinical benefit of bevacizumab in metastatic colorectal cancer is independent of K-ras mutation status: analysis of a phase III study of bevacizumab with chemotherapy in previously untreated metastatic colorectal cancer. Oncologist. 2009;14:22–8.
Price TJ, Hardingham JE, Lee CK, Weickhardt A, Townsend AR, Wrin JW, et al. Impact of KRAS and BRAF gene mutation status on outcomes from the phase III AGITG MAX trial of capecitabine alone or in combination with bevacizumab and mitomycin in advanced colorectal cancer. J Clin Oncol. 2011;29:2675–82.
Kubicka S, Greil R, André T, Bennouna J, Sastre J, Van Cutsem E, et al. Bevacizumab plus chemotherapy continued beyond first progression in patients with metastatic colorectal cancer previously treated with bevacizumab plus chemotherapy: ML18147 study KRAS subgroup Findings. Ann Oncol. 2013;24:2342–9.
Passardi A, Scarpi E, Cavanna L, Fontana A, Vertogen B, Ruscelli S, et al. Effectiveness of bevacizumab added to gold standard chemotherapy in metastatic colorectal cancer (mCRC): final results from the Itaca randomized clinical trial. J Clin Oncol 31(Suppl):Abstract 3517; 2013.
Acknowledgments
This study was supported by an unrestricted grant from Roche Farma S.A. The study sponsors had no involvement in the study design, collection, analysis and interpretation of data or in the decision to submit the manuscript for publication, but provided support for third-party writing assistance of this manuscript.
Conflict of interest
Santiago Lopez-Ben has received research funding from Roche. Rosa Maria Ortiz-Duran has received research funding from Roche. Esther Diaz has received research funding from Roche. Angels Quera has received research funding from Roche. Joan Figueras has a consultancy role for Roche, has received honoraria for speaking from Roche and Merck, and has received research funding from Roche. Ruth Vera, Marisa Gomez Dorronsoro, Antonio Viudez, Bernardo Queralt, Irene Hernandez, Cruz Zazpe, Jordi Soriano, Irene Amat, Javier Herrera Cabezón, Antoni Codina-Barreras and Xavi Hernandez-Yagüe have nothing to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vera, R., Gomez Dorronsoro, M., Lopez-Ben, S. et al. Retrospective analysis of pathological response in colorectal cancer liver metastases following treatment with bevacizumab. Clin Transl Oncol 16, 739–745 (2014). https://doi.org/10.1007/s12094-013-1142-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-013-1142-x