Abstract
Background
The diagnostic and prognostic values of glypican3 (GPC3) and glutamine synthetase (GS) proteins in hepatocellular carcinoma (HCC) have been reported, but their specificity and sensitivity remain low. Here, we applied RNAscope to improve HCC early pathological and differential diagnosis by estimating GPC3 and GS mRNAs.
Methods
We performed RNAscope and immunohistochemistry (IHC) to detect GPC3 and GS biomarkers on the tissue sections of 194 cases, including high- and low-grade liver dysplastic nodules; highly, moderately, and poorly differentiated HCCs; intrahepatic cholangiocarcinomas (ICCs); metastatic HCC; and carcinomas from other organs.
Results
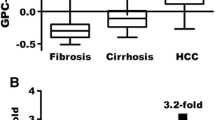
The results showed that all the cases that were negative for GPC3 by RNAscope were also negative for this protein by IHC. The use of RNAscope assay improved the GPC3 and GS specificity and sensitivity by 20–30%. Hence, HCC shows early recognition and upgrades the metastatic HCC differentiation by 23% compared with IHC (p = 0.0001, 0.0064). Meanwhile, all liver cirrhosis, cholangiocytes and non-HCC samples were negative for GPC3 and GS except lymphocytes in lymphomas, and 2 (8.3%) out of the 24 ICC samples but not in the cancer cells.
Conclusion
RNAscope for GPC3 and GS panel was highly specific and sensitive for the pathological identification of dysplastic nodules, early stages of HCCs, and would differentiate them from HCCs and metastatic tumors compared with IHC.





Similar content being viewed by others
Data availability
The authors declare that all the data supporting the findings of this study are available within the article and its supplementary information files.
Abbreviations
- GPC3:
-
Glypican3
- GS:
-
Glutamine synthetase
- HCC:
-
Hepatocellular carcinoma
- IHC:
-
Immunohistochemistry
- ICCs:
-
Intrahepatic cholangiocarcinomas
- HBV:
-
Hepatitis B virus
- WHO:
-
World Health Organization
- HCV:
-
Hepatitis C virus
- ANOVA:
-
Analysis of variance
- SEM:
-
Standard error of mean
References
Fitzmorris P, Singal AK. Surveillance and diagnosis of hepatocellular carcinoma. Gastroenterol Hepatol. 2015;11(1):38.
Bakheet AMH, Mahmoud SA, Huang Y, Zhang J, Wang J, Wei Y, Gamallat Y, et al. Ezrin as a possible diagnostic and/or prognostic biomarker in mice lymphatic metastatic hepatocellular carcinoma in vivo. BioFactors. 2017;43(5):662–72.
Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450–62.
Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–2.
Ghouri YA, Mian I, Rowe JH. Review of hepatocellular carcinoma: epidemiology, etiology, and carcinogenesis. J Carcinog. 2017;16:1.
Kondo F, Fukusato T, Kudo M. Pathological diagnosis of benign hepatocellular nodular lesions based on the new World Health Organization classification. Oncology. 2014;87(Suppl 1):37–49.
Schlageter M, Terracciano LM, D’Angelo S, Sorrentino P. Histopathology of hepatocellular carcinoma. World J Gastroenterol. 2014;20(43):15955–64.
Agni RM. Diagnostic histopathology of hepatocellular carcinoma: a case-based review. Semin Diagn Pathol. 2017;34(2):126–37.
Choi WT, Kakar S. Immunohistochemistry in the diagnosis of hepatocellular carcinoma. Gastroenterol Clin N Am. 2017;46(2):311–25.
Anderson CM, Zhang B, Miller M, Butko E, Wu X, Laver T, Kernag C, et al. Fully automated RNAscope in situ hybridization assays for formalin-fixed paraffin-embedded cells and tissues. J Cell Biochem. 2016;117(10):2201–8.
Wang F, Flanagan J, Su N, Wang LC, Bui S, Nielson A, Wu X, et al. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn. 2012;14(1):22–9.
Hamilton SR, Aaltonen LA. Pathology and genetics of tumours of the digestive system. International Agency for Research on Cancer (IARC) 69372 Lyon, France; 2000. pp.155–216.
Ou-Yang RJ, Hui P, Yang XJ, Zynger DL. Expression of glypican 3 in placental site trophoblastic tumor. Diagn Pathol. 2010;5:64.
Zhang J, Zhang M, Ma H, Song X, He L, Ye X, et al. Overexpression of glypican-3 is a predictor of poor prognosis in hepatocellular carcinoma: an updated meta-analysis. Medicine. 2018;97(24):e11130.
Behne T, Copur MS. Biomarkers for hepatocellular carcinoma. Int J Hepatol. 2012;2012:859076.
Ofuji K, Saito K, Suzuki S, Shimomura M, Shirakawa H, Nobuoka D, Sawada Y, et al. Perioperative plasma glypican-3 level may enable prediction of the risk of recurrence after surgery in patients with stage I hepatocellular carcinoma. Oncotarget. 2017;8(23):37835–44.
Long J, Wang H, Lang Z, Wang T, Long M, Wang B. Expression level of glutamine synthetase is increased in hepatocellular carcinoma and liver tissue with cirrhosis and chronic hepatitis B. Hepatol Int. 2011;5(2):698–706.
Loeppen S, Schneider D, Gaunitz F, Gebhardt R, Kurek R, Buchmann A, et al. Overexpression of glutamine synthetase is associated with β-catenin-mutations in mouse liver tumors during promotion of hepatocarcinogenesis by phenobarbital. Cancer Res. 2002;62:5685–8.
Uthamalingam P, Das A, Behra A, Kalra N, Chawla Y. Diagnostic value of glypican3, heat shock protein 70 and glutamine synthetase in hepatocellular carcinoma arising in cirrhotic and non-cirrhotic livers. J Clin Exp Hepatol. 2018;8(2):173–80.
Di Tommaso L, Franchi G, Park YN, Fiamengo B, Destro A, Morenghi E, Montorsi M, et al. Diagnostic value of HSP70, glypican 3, and glutamine synthetase in hepatocellular nodules in cirrhosis. Hepatology. 2007;45(3):725–34.
Tsuchiya N, Sawada Y, Endo I, Saito K, Uemura Y, Nakatsura T. Biomarkers for the early diagnosis of hepatocellular carcinoma. World J Gastroenterol. 2015;21(37):10573–83.
Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. 3rd ed. WHO Press; 2010.
Pace CN, Trevino S, Prabhakaran E, Scholtz JM. Protein structure, stability and solubility in water and other solvents. Philos Trans R Soc Lond B Biol Sci. 2004;359(1448):1225–34 (discussion 1234–1235).
Suzuki M, Sugimoto K, Tanaka J, Tameda M, Inagaki Y, Kusagawa S, Nojiri K, et al. Up-regulation of glypican-3 in human hepatocellular carcinoma. Anticancer Res. 2010;30(12):5055–61.
Fernandez D, Guereno M, Lago Huvelle MA, Cercato M, Peters MG. Signaling network involved in the GPC3-induced inhibition of breast cancer progression: role of canonical Wnt pathway. J Cancer Res Clin Oncol. 2018;144(12):2399–418.
Han S, Ma X, Zhao Y, Zhao H, Batista A, Zhou S, Zhou X, et al. Identification of glypican-3 as a potential metastasis suppressor gene in gastric cancer. Oncotarget. 2016;7(28):44406–16.
Castegna A, Menga A. Glutamine synthetase: localization dictates outcome. Genes. 2018;9(2):108.
Wang HL, Florencia A, Zhai QJ, Adley B, Chuang ST, Yang XJ. Glypican-3 as a useful diagnostic marker that distinguishes hepatocellular carcinoma from benign hepatocellular mass lesions. Arch Pathol Lab Med. 2008;132:1723–8.
Yang SL, Fang X, Huang ZZ, Liu XJ, Xiong ZF, Liu P, Yao HY, et al. Can serum glypican-3 be a biomarker for effective diagnosis of hepatocellular carcinoma? A meta-analysis of the literature. Dis Markers. 2014;2014:127831.
Wong MC, Jiang JY, Goggins WB, Liang M, Fang Y, Fung FD, Leung C, et al. International incidence and mortality trends of liver cancer: a global profile. Sci Rep. 2017;7:45846.
Acknowledgements
We gratefully acknowledge the support from the National Natural Science Foundation of China (No. 81572309), the Natural Science Foundation of Guangdong Province (No. 2017A030313502 and No. 2018A030313650 and No. 2019A1515011455), and the Guangzhou Science and Technology Project (No. 201707010119), Guangdong Province, China.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81572309), the Natural Science Foundation of Guangdong Province (No. 2017A030313502 and No. 2018A030313650 and No. 2019A1515011455), and the Guangzhou Science and Technology Project (No. 201707010119), Guangdong Province, China. The authors thank the Department of Pathology of The Third Affiliated Hospital of Sun Yat-sen University.
Author information
Authors and Affiliations
Contributions
CKS contributed to study concept, design and supervised all works; AMHB contributed to the experiments, analysis, and interpretation of data and drafting of the manuscript. CZ, JNC contributed to samples’ pathological diagnosis and staging. JYZ contributed to acquisition of data. JTH, YD, LPG, and YHB contributed to technical and material support.
Corresponding author
Ethics declarations
Conflict of interest
Ahmed Musa Hago Bakheet, Chang Zhao, Jian-Ning Chen, Jing-Yue Zhang, Jun-Ting Huang, Yu Du, Li-Ping Gong, Yuan-Hua Bi, and Chun-Kui Shao declare that no conflicts of interest exist. The authors have no financial relationship to disclose.
Ethical approval
We declare that the authors used the archived wax-embedded blocks of liver samples in the Pathology Department at the Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, China. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This article did not show patients’ personal information and the medical ethics involved in the research were examined and approved by the Ethics Committee of Sun Yat-sen University, Guangzhou, China. None of the patients treated by chemotherapy or radiotherapy before the operation and the medical ethics involved in the research were examined and approved by the Ethics Committee of Sun Yat-sen University, Guangzhou, China.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chang Zhao is the co-first author.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bakheet, A.M.H., Zhao, C., Chen, JN. et al. Improving pathological early diagnosis and differential biomarker value for hepatocellular carcinoma via RNAscope technology. Hepatol Int 14, 96–104 (2020). https://doi.org/10.1007/s12072-019-10006-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-019-10006-z