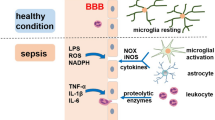
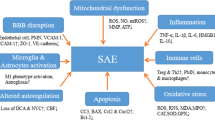
Abstract
Sepsis is an organ dysfunction caused by an uncontrolled inflammatory response from the host to an infection. Sepsis is the main cause of morbidity and mortality in intensive care units (ICU) worldwide. One of the first organs to suffer from injuries resulting from sepsis is the brain. The central nervous system (CNS) is particularly vulnerable to damage, mediated by inflammatory and oxidative processes, which can cause the sepsis-associated encephalopathy (SAE), being reported in up to 70% of septic patients. This review aims to bring a summary of the main pathophysiological changes and dysfunctions in SAE, and the main focuses of current experimental studies for new treatments and therapies. The pathophysiology of SAE is complex and multifactorial, combining intertwined processes, and is promoted by countless alterations and dysfunctions resulting from sepsis, such as inflammation, neuroinflammation, oxidative stress, reduced brain metabolism, and injuries to the integrity of the blood-brain barrier (BBB). The treatment is limited once its cause is not completely understood. The patient’s sedation is far to provide an adequate treatment to this complex condition. Studies and experimental advances are important for a better understanding of its pathophysiology and for the development of new treatments, medicines, and therapies for the treatment of SAE and to reduce its effects during and after sepsis.

Similar content being viewed by others
Data Availability
Not applicable.
Abbreviations
- SAE:
-
Sepsis-associated encephalopathy
- ICU:
-
Intensive care units
- CNS:
-
Central nervous system
- BBB:
-
Blood-brain barrier
- TNF-α:
-
Necrosis factor alpha
- IL:
-
Interleukins
- CSF:
-
Cerebrospinal fluid
- ROS:
-
Reactive oxygen species
- NO:
-
Nitric oxide
- RNS:
-
Reactive nitrogen species
- H2O2 :
-
Hydrogen peroxide
- O2 . :
-
Superoxide
- .OH:
-
Hydroxyl
- NO2 . :
-
Nitrogen dioxide
- ATP:
-
Adenosine triphosphate
- CLP:
-
Cecal ligation puncture procedure
- FBP:
-
Fructose-1,6-bisphosphate
- 18F-FDG:
-
18F-fluoro-2-deoxy-d-glucose
- GSDMD:
-
Gasdermin-D protein
- CAT:
-
Catalase
- GPx:
-
Glutathione peroxidase
- KYN:
-
Kynurenine
- SOD:
-
Superoxide dismutase
- EE:
-
Ecballium elaterium
- FBP:
-
Fructose-1,6-bisphosphate
- Ngb:
-
Neuroglobin
- FO:
-
Fish oil
- IVIg:
-
Immunoglobulin
References
Shankar-Hari M, Phillips GS, Levy ML, Seymour CW, Liu VX, Deutschman CS et al (2016) Developing a newdefinition and assessing newclinical criteria for Septic shock: for the third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA - J Am Med Assoc 315(8):775–787
Gotts JE, Matthay MA (2016) Sepsis: pathophysiology and clinical management. BMJ 353:1–20
Delano MJ, Ward PA (2016) Sepsis-induced immune dysfunction: can immune therapies reduce mortality? J Clin Invest 126(1):23–31
Schulte W, Bernhagen J, Bucala R (2013) Cytokines in sepsis: potent immunoregulators and potential therapeutic targets - an updated view. Mediators Inflamm 2013.
Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM et al (2013) Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 39(2):165–228
Shankar-Hari M, Phillips GS, Levy ML, Seymour CW, Liu VX, Deutschman CS et al (2016) Developing a new definition and assessing new clinical criteria for septic shock: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA - J Am Med Assoc 315(8):775–787
Cossart YE (2014) The rise and fall of infectious diseases: Australian perspectives, 1914-2014. Med J Aust 201(1 Suppl):11–14
Nedeva C, Menassa J, Puthalakath H (2019) Sepsis: inflammation is a necessary evil. Front Cell Dev Biol 7(JUN):1–12.
Fleischmann C, Scherag A, Adhikari NKJ, Hartog CS, Tsaganos T, Schlattmann P et al (2016) Assessment of global incidence and mortality of hospital-treated sepsis current estimates and limitations. Am J Respir Crit Care Med 193(3):259–272
Wang TL (2014) Prolonged neuroinflammation after lipopolysaccharide exposure in aged rats. PLoS One 9(8)
Michelon C, Michels M, Abatti M, Vieira A, Borges H, Dominguini D et al (2020) The role of secretase pathway in long-term brain inflammation and cognitive impairment in an animal model of severe sepsis. Mol Neurobiol 57(2):1159–1169
Sankowski R, Mader S, Valdés-Ferrer SI (2015) Systemic inflammation and the brain: novel roles of genetic, molecular, and environmental cues as drivers of neurodegeneration. Front Cell Neurosci 9(FEB):1–20.
Widmann CN, Heneka MT (2014) Long-term cerebral consequences of sepsis. Lancet Neurol 13(6):630–636
Sonneville R, Verdonk F, Rauturier C, Klein IF, Wolff M, Annane D (2013) Understanding brain dysfunction in sepsis. Ann Intensive Care 3(Figure 1):15.
Kozlov AV, Bahrami S, Redl H, Szabo C (2017) Alterations in nitric oxide homeostasis during traumatic brain injury. Biochim Biophys Acta - Mol Basis Dis 1863(10):2627–2632
Robba C, Crippa IA, Taccone FS (2018) Septic encephalopathy. Curr Neurol Neurosci Rep 18(82)
Taccone FS, Scolletta S, Franchi F, Donadello K, Oddo M (2013) Brain perfusion in sepsis. Curr Vasc Pharm 11(2):170–186
Young GB (2013) Encephalopathy of infection and systemic inflammation. J Clin Neurophysiol 30(5):454–461
Andonegui G, Zelinski EL, Schubert CL, Knight D, Craig LA, Winston BW et al (2018) Targeting inflammatory monocytes in sepsis-associated encephalopathy and long-term cognitive impairment. JCI insight 3(9):1–20
Mazeraud A, Pascal Q, Verdonk F, Heming N, Chrétien F, Sharshar T (2016) Neuroanatomy and physiology of brain dysfunction in sepsis. Clin Chest Med 37(2):333–345
Adam N, Kandelman S, Mantz J (2013) Sepsis-induced brain dysfunction. Expert Rev Anti Infect Ther 11(2):211–221
Tian M, Qingzhen L, Zhiyang Y, Chunlong C, Jiao D, Zhang L et al (2019) Attractylone attenuates sepsis-associated encephalopathy and cognitive dysfunction by inhibiting microglial activation and neuroinflammation. J Cell Biochem 120(5):7101–7108
Michels M, Ávila P, Pescador B, Vieira A, Abatti M, Cucker L et al (2019) Microglial cells depletion increases inflammation and modifies microglial phenotypes in an animal model of severe sepsis. Mol Neurobiol 56(11):7296–7304
Shulyatnikova T, Verkhratsky A (2019) Astroglia in sepsis associated encephalopathy. Neurochem Res 45(1):83–99
Hoogland ICM, Houbolt C, van Westerloo DJ, van Gool WA, van de Beek D (2015) Systemic inflammation and microglial activation: systematic review of animal experiments. J Neuroinflammation 12(1):1–13
Lemstra AW, Cm J, Hoozemans JJM, Van Haastert ES, Rozemuller AJM, Eikelenboom P et al (2007) Microglia activation in sepsis: a case-control study. J Neuroinflammation 15(4):1–8
Danielski LG, Giustina A Della, Goldim MP, Florentino D, Mathias K, Garbossa L, et al. (2018) Vitamin B6 reduces neurochemical and long-term cognitive alterations after polymicrobial sepsis: involvement of the kynurenine pathway modulation. Mol Neurobiol 55(6):5255–5268
Bedirli N, Bagriacik EU, Yilmaz G, Ozkose Z, Kavutçu M, Cavunt Bayraktar A et al (2018) Sevoflurane exerts brain-protective effects against sepsis-associated encephalopathy and memory impairment through caspase 3/9 and Bax/Bcl signaling pathway in a rat model of sepsis. J Int Med Res 46(7):2828–2842
Danielski LG, Giustina A Della, Badawy M, Barichello T, Quevedo J, Dal-Pizzol F, et al. (2018) Brain barrier breakdown as a cause and consequence of neuroinflammation in sepsis. Mol Neurobiol 55(2):1045–1053
Nwafor DC, Brichacek AL, Mohammad AS, Griffith J, Lucke-Wold BP, Benkovic SA et al (2019) Targeting the blood-brain barrier to prevent sepsis-associated cognitive impairment. J Cent Nerv Syst Dis 11:117957351984065
Bookheimer SY, Strojwas MH et al (2000) Patterns of brain activation in people at risk for Alzheimer’s disease. N Eng J Med 343(7):450–456
Reiman EM, Caselli RJ, Chen K, Alexander GE, Bandy D, Frost J (2001) Declining brain activity in cognitively normal apolipoprotein epsilon 4 heterozygotes: a foundation for using positron emission tomography to efficiently test treatments to prevent Alzheimer’s disease. Proc Natl Acad Sci USA 98(6)
Cunnane S, Ph D, Nugent S, Sc B, Roy M, Sc M, et al. (2011) Brain fuel metabolism , aging , and Alzheimer’s disease. Nutrition 27(1):3–20.
Catarina A V, Luft C, Greggio S, Venturin GT, Ferreira F, Marques EP, et al. (2018) Fructose-1,6-bisphosphate preserves glucose metabolism integrity and reduces reactive oxygen species in the brain during experimental sepsis. Brain Res 1698:54–61.
Dantzer R, Kelley KW (2008) From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 9:46–56
Garcia J, Kimeldorf DJ, Koelling RA (1955) Conditioned aversion to saccharin resulting from exposure to gamma radiation. Science 122:157–158
Gamache FW Jr DT (1982) Alterations in neurological function in head-injured patients experiencing major episodes of sepsis. Neurosurgery 10(2):468–472.
Calsavara AJC, Nobre V, Barichello T, Teixeira AL (2018) Post-sepsis cognitive impairment and associated risk factors: a systematic review. Aust Crit Care 31(4):242–253
Ebersoldt M, Sharshar T, Annane D (2007) Sepsis-associated delirium. Intensive Care Med 33(6):941–950
Leon A, Lepousé C, Floch T, Graftieaux J (2006) Agression cérébrale au cours du sepsis sévère Brain injury during severe sepsis. Ann Fr Anesth Reanim 25(8):863–867
Dittus RS, Thomason JWW, Jackson JC, Shintani ÃAK, Ely EW, Mph à (2006) Delirium and its motoric subtypes: a study of 614 critically ill patients. J Am Geriatr Soc 54(3):479–484.
Barichello T, Sayana P, Giridharan VV, Arumanayagam AS, Narendran B, Giustina A Della, et al. (2019) Long-term cognitive outcomes after sepsis: a translational systematic review. Mol Neurobiol 56(1):186–251
Semmler A, Widmann CN, Okulla T, Urbach H, Kaiser M, Widman G, et al. (2013) Persistent cognitive impairment , hippocampal atrophy and EEG changes in sepsis survivors. J Neurol Neurosurg Psychiatry 84(1):62–69.
Li W, Wang Y, Wang X, He Z, Liu F, Zhi W et al (2016) Esculin attenuates endotoxin shock induced by lipopolysaccharide in mouse and NO production in vitro through inhibition of NF-κB activation. Eur J Pharmacol 791(76):726–734
Qin X, Jiang X, Jiang X, Wang Y, Miao Z, He W (2016) Micheliolide inhibits LPS-induced inflammatory response and protects mice from LPS challenge. Sci Rep :1–13.
He H, Geng T, Chen P, Wang M, Hu J, Kang L et al (2016) OPEN NK cells promote neutrophil recruitment in the brain during sepsis-induced neuroinflammation. Sci Rep 6:1–14
Sharshar T, Gray F, Lorin G, Grandmaison D, Hopkinson NS, Ross E et al (2003) Mechanisms of disease Apoptosis of neurons in cardiovascular autonomic centres triggered by inducible nitric oxide synthase after death from septic shock. Lancet 362(9398):1799–1805
Alexander JJ, Jacob A et al (2008) TNF is a key mediator of septic encephalopathy acting through its receptor, TNF receptor-1. Neurochem int 52(3):447–456
Rorato R, Menezes AM, Giusti-paiva A, De Castro M (2009) Prostaglandin mediates endotoxaemia-induced hypophagia by activation of pro-opiomelanocortin and corticotrophin-releasing factor neurons in rats. Exp Physiol 94(3):371–379
Rump K, Adamzik M (2018) Function of aquaporins in sepsis: a systematic review. Cell Biosci 8:1–7
Takatani Y, Ono K (2018) Inducible nitric oxide synthase during the late phase of sepsis is associated with hypothermia and immune cell migration. Lab Investig 98(5):629–639
Cunningham C, Maclullich AMJ (2013) At the extreme end of the psychoneuroimmunological spectrum: delirium as a maladaptive sickness behaviour response. Brain Behav Immun 28:1–13
Westhoff D, Engelen-Lee JY, Hoogland ICM, Aronica EMA, Van Westerloo DJ, Van De Beek D et al (2019) Systemic infection and microglia activation: a prospective postmortem study in sepsis patients. Immun Ageing 16(1):1–10
Halliwell B (2006) Oxidative stress and neurodegeneration: where are we now? J Neurochem 97(6):1634–1658
Brookes PS, Bolan JP, Y SJRH (1999) The assumption that nitric oxide inhibits mitochondrial ATP synthesis is correct. FEBS Lett 446(2–3):261–263.
Berg RMG, Møller K, Bailey DM (2011) Neuro-oxidative-nitrosative stress in sepsis. J Cereb Blood Flow Metab 31(7):1532–1544
Cassina A, Radi R (1996) Differential inhibitory action of nitric oxide and peroxynitrite on mitochondrial electron transport. Arch Biochem Biophys 328(2):309–316
Tang G, Yang H, Chen J, Shi M, Ge L (2017) Metformin ameliorates sepsis-induced brain injury by inhibiting apoptosis, oxidative stress and neuroinflammation via the PI3K/Akt signaling pathway. Oncotarget 8(58):97977–97989.
Zhu S, Huang W, Huang L, Han Y, Han Q (2016) Huperzine A protects sepsis associated encephalopathy by promoting the deficient cholinergic nervous function. Neurosci Lett 631:70–78
Semmler A, Okulla T, Sastre M, Dumitrescu-ozimek L, Heneka MT (2005) Systemic inflammation induces apoptosis with variable vulnerability of different brain regions. J Chem Neuroanat 30:144–157
Brain T The expensive-tissue. 36(2).
Tsacopoulos M, Magistretti PJ (1996) Metabolic coupling between glia and neurons. J Neurosci. 16(3):877–885
Shulman RG, Hyder F, Rothman DL (2001) Cerebral energetics and the glycogen shunt: neurochemical basis of functional imaging. Proc Natl Acad Sci USA 98(11):6417–6422
Gofton TE, Young GB (2012) Sepsis-associated encephalopathy. Nat Rev Neurol 8(October):557–566
Oddo M, Taccone FS (2015) How to monitor the brain in septic patients? Minerva Anestesiol 81(7):776–788
Taccone FS, Castanares-Zapater, et al. (2010) Cerebral autoregulation is influenced by carbon dioxide levels in patients with septic shock. Neurocrit Care. 12(1):35–42
Semmler A, Hermann S (2008) Sepsis causes neuroinflammation and concomitant decrease of cerebral metabolism. J Neuroinflammation 5:38
Everson-Rose SA, Ryan JP (2015) Diabetes, obesity, and the brain: new developments in biobehavioral medicine. Psychosom Med 77(6):612–615
Lin A-L, Parikh I, Hoffman JD, Ma D (2017) Neuroimaging biomarkers of caloric restriction on brain metabolic and vascular functions. Curr Nutr Rep 6(1):41–48
Bangen KJ, Beiser A, Delano-Wood L, Nation DA, Lamar M, Libon DJ et al (2013) APOE genotype modifies the relationship between midlife vascular risk factors and later cognitive decline. J Stroke Cerebrovasc Dis 22(8):1361–1369
Cunnane S, Nugent S, Roy M, Courchesne-Loyer A, Croteau E, Tremblay S et al (2012) Brain fuel metabolism, aging, and Alzheimer’s disease. Nutrition 27(1):3–20
Keaney J, Campbell M (2015) The dynamic blood–brain barrier. FEBS J 282(21):4067–4079.
Engelhardt B, Sorokin L (2009) The blood–brain and the blood–cerebrospinal fluid barriers: function and dysfunction. Semin Immunopathol 31(4):497–511.
Weighardt H, Holzmann B (2008) Role of Toll-like receptor responses for sepsis pathogenesis. Immunobiology 212(9–10):715–722
Janeway CA, Medzhitov R (2002) Innate immune recognition. Annu Rev Immunol 20(2):197–216
Junior CAJ (2001) How the immune system protects the host from infection. Microbes Infect 3(13):1167–1171
Chapouly C, Argaw AT, Horng S, Castro K, Zhang J, Asp L, et al. (2015) Astrocytic TYMP and VEGFA drive blood–brain barrier opening in inflammatory central nervous system lesions. Brain 138(6):1548–1567.
Vincent VAM, Tilders FJH, Dam AVAN (1997) Inhibition of endotoxin-induced nitric oxide synthase production in microglial cells by the presence of astroglial cells: a role for transforming growth factor. Glia 19(3):190–198.
Lee SC (1993) Cytokine production by human fetal microglia and astrocytes. Differential induction by lipopolysaccharide and IL-1 beta. J Immunol 150(7):2659–2667
Geissmann F, Markus G, Manz SJ, Sieweke MH, Merad M, Ley K (2010) Development of monocytes, macrophages, and dendritic cells. Science 327(5966):656–661
Opal SM, Ellis JL, Suri V, Freudenberg JM, Vlasuk GP, Li Y et al (2016) Pharmacological SIRT1 activation improves mortality and markedly alters transcriptional profiles that accompany experimental sepsis. Shock 45(4):411–418
Zhao L, An R, Yang Y, Yang X, Liu H, Yue L et al (2015) Melatonin alleviates brain injury in mice subjected to cecal ligation and puncture via attenuating in fl ammation, apoptosis, and oxidative stress: the role of SIRT1 signaling. J Pineal Res 59(2):230–239
Hernández-Jiménez M, Hurtado O, Cuartero MI, Ballesteros I, Moraga A, Pradillo JM et al (2013) Silent information regulator 1 protects the brain against cerebral ischemic damage. Stroke 44(8):2333–2337
Bai XZ, He T, Gao JX, Liu Y, Liu JQ, Han SC et al (2016) Melatonin prevents acute kidney injury in severely burned rats via the activation of SIRT1. Sci Rep 6(August):1–13
Zhu Y, Wang K, Ma Z, Liu D, Yang Y, Sun M et al (2019) SIRT1 activation by butein attenuates sepsis-induced brain injury in mice subjected to cecal ligation and puncture via alleviating inflammatory and oxidative stress. Toxicol Appl Pharmacol 363:34–46
Lee D, Jeong G (2016) Butein provides neuroprotective and anti-neuroin fl ammatory effects through expression by activating the PI3K/Akt pathway Tables of Links. Br J Pharmacol 173(19):2894–2909.
Padmavathi G, Kishor N, Bordoloi D, Arfuso F, Mishra S, Sethi G, et al. (2017) Phytomedicine butein in health and disease: a comprehensive review. Phytomedicine 25:118–127.
Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H et al (2015) Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 562:660–665
Kayagaki N, Stowe IB, Lee BL, Rourke KO, Anderson K, Warming S et al (2015) Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526:666–671
Khan M, Shah SA, Kim MO (2018) 17β-estradiol via SIRT1/acetyl-p53/NF-kB signaling pathway rescued postnatal rat brain against acute ethanol intoxication. Mol Neurobiol 55(4):3067–3078
Kou D-Q, Jiang Y-L, Qin J-H, Huang Y-H (2017) Magnolol attenuates the inflammation and apoptosis through the activation of SIRT1 in experimental stroke rats. Pharmacol Rep 69(4):642–647
Al. P. BA. Z et (2016) Orexin activation counteracts decreases in nonexercise activity thermogenesis (NEAT) caused by high fat diet. Physiol Behav 176(1):139–148.
Xu X e., Liu L, Wang Y chang, Wang C tao, Zheng Q, Liu Q xin, et al. (2019) Caspase-1 inhibitor exerts brain-protective effects against sepsis-associated encephalopathy and cognitive impairments in a mouse model of sepsis. Brain Behav Immun 80(January):859–870
Wang P, Hu Y, Yao D, Li Y (2018) Omi/HtrA2 regulates a mitochondria-dependent apoptotic pathway in a murine model of septic encephalopathy. Cell Physiol Biochem 49(6):2163–2173
Lacroix-desmazes S, Kazatchkine MD, Kaveri SV (2005) Intravenous immunoglobulin in neurological disorders: a mechanistic perspective. J Neurol 252:1–6
Esen F, Ozcan PE, Tuzun E, Boone MD (2018) Mechanisms of action of intravenous immunoglobulin in septic encephalopathy. Rev Neurosci 29(4):417–423
Brunet A, Datta SR, Greenberg ME (2001) Transcription-dependent and -independent control of neuronal survival by the PI3K–Akt signaling pathway. Curr Opin Neurobiol 11(3):297–305.
Burmester T, Weich B, Reinhardt S (2000) A vertebrate globin expressed in the brain. 407(September):1998–2001.
Raida Z, Hundahl CA, Nyengaard JR, Hay-schmidt A (2013) Neuroglobin over expressing mice: expression pattern and effect on brain ischemic infarct size. PLoS One 8(10):e 76565.
Zhang LN, Ai YH, Gong H, Guo QL, Huang L, Liu ZY YB (2014) Expression and role of neuroglobin in rats with sepsis-associated encephalopathy*. Crit Care Med 42(1):e 12-21.
Deng S, Ai Y, Gong H, Chen C, Peng Q, Huang L et al (2017) Neuroglobin protects rats from sepsis-associated encephalopathy via a PI3K/Akt/Bax-dependent mechanism. J Mol Neurosci 63(1):1–8
Campbell BM, Charych E, Lee AW, Möller T, Dantzer R (2014) Kynurenines in CNS disease: regulation by inflammatory cytokines. Front Neurosci 8(February):1–22
Maddison DC, Giorgini F (2015) The kynurenine pathway and neurodegenerative disease. Semin Cell Dev Biol 40:134–141
Bordignon Nunes F, Simões Pires MG, Alves Filho JCF, Wächter PH, De Oliveira JR (2002) Physiopathological studies in septic rats and the use of fructose 1,6-bisphosphate as cellular protection. Crit Care Med 30(9):2069–2074
Pedrazza L, Lunardelli A, Luft C, Cruz CU, De Mesquita FC, Bitencourt S et al (2014) Mesenchymal stem cells decrease splenocytes apoptosis in a sepsis experimental model. Inflamm Res 63(9):719–728
Moataz E, El B, Chalupov M, Pra G, Suchý P (2015) Hepatoprotective and proapoptotic effect of Ecballium elaterium on CCl4-induced hepatotoxicity in rats. Asian Pac J Trop Med 8(7):526–531
Uslu C, Karasen RM, Sahin F (2006) Effect of aqueous extracts of Ecballium elaterium rich, in the rabbit model of rhinosinusitis. Int J Pediatr Otorhinolaryngol 70(3):515–518
Arslan D, Ekinci A, Arici A, Bozdemir E, Akil E, Ozdemir HH (2017) Effects of Ecballium elaterium on brain in a rat model of sepsis-associated encephalopathy. Libyan J Med 12(1)
Sc DM, Della A, Ph G, Pereira M, Ph G, Eduarda M et al (2020) Fish oil À rich lipid emulsion modulates neuroin fl ammation and prevents long-term cognitive dysfunction after sepsis. Nutrition 70:1–9
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—finance code 001.
Author information
Authors and Affiliations
Contributions
All authors have been involved in drafting the manuscript or revising it critically for important intellectual content.
Corresponding author
Ethics declarations
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Consent to Participate
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Catarina, A.V., Branchini, G., Bettoni, L. et al. Sepsis-Associated Encephalopathy: from Pathophysiology to Progress in Experimental Studies. Mol Neurobiol 58, 2770–2779 (2021). https://doi.org/10.1007/s12035-021-02303-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02303-2