Abstract
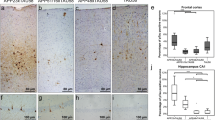
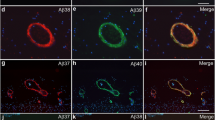
The epsilon 4 allele of the apolipoprotein E (ApoE4) gene is the most important risk factor implicated in Alzheimer’s disease (AD) etiology. ApoE4 is more susceptible to proteolysis, and ApoE fragments have been shown to promote tau hyperphosphorylation and exert neurotoxic properties. While a plethora of studies deals with the effect of ApoE and its fragments on amyloid-β peptide (Aβ) deposition and clearance, it is largely unknown whether Aβ in turn influences human or murine ApoE expression and its proteolysis. The present study is the first to show that endogenous murine ApoE becomes proteolytically processed in a way reminiscent of human ApoE fragmentation in different AD mouse models, including APP/PS1KI or 5XFAD. Murine ApoE fragments were demonstrated to accumulate mainly in synaptic fractions in AD mouse models. In vitro experiments, as well as analysis of mouse models at different time points, suggest that the amount of total ApoE is associated with extracellular Aβ while the amount of its fragments is linked to intracellular Aβ levels. Murine ApoE fragmentation is a common feature in different AD transgenic mouse models and could be directly associated with intraneuronal Aβ accumulation. Extracellular amyloid induces an elevation in full-length ApoE expression, which might present a protective mechanism toward Aβ clearance. The demonstrated fragments of murine ApoE in vitro and in vivo might therefore play a crucial role in the progression of AD pathology in murine AD models.







Similar content being viewed by others
References
Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL et al (1993) Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261(5123):921–923
Raber J, Huang Y, Ashford JW (2004) ApoE genotype accounts for the vast majority of AD risk and AD pathology. Neurobiol Aging 25(5):641–650. doi:10.1016/j.neurobiolaging.2003.12.023
Holtzman DM, Herz J, Bu G (2012) Apolipoprotein E and Apolipoprotein E Receptors: Normal Biology and Roles in Alzheimer Disease. Cold Spring Harbor Perspectives in Medicine 2 (3). doi:10.1101/cshperspect.a006312
Kanekiyo T, Xu H, Bu G (2014) ApoE and Abeta in Alzheimer s disease: accidental encounters or partners? Neuron 81(4):740–754
Castellano JM, Kim J, Stewart FR, Jiang H, Demattos RB, Patterson BW, Fagan AM, Morris JC et al (2011) Human apoE isoforms differentially regulate brain amyloid-{beta} peptide clearance. Sci Transl Med 3(89):89ra57. doi:10.1126/scitranslmed.3002156
Mahley RW (1988) Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science 240(4852):622–630
Bao F, Arai H, Matsushita S, Higuchi S, Sasaki H (1996) Expression of apolipoprotein E in normal and diverse neurodegenerative disease brain. Neuroreport 7(11):1733–1739
Han SH, Einstein G, Weisgraber KH, Strittmatter WJ, Saunders AM, Pericak-Vance M, Roses AD, Schmechel DE (1994) Apolipoprotein E is localized to the cytoplasm of human cortical neurons: a light and electron microscopic study. J Neuropathol Exp Neurol 53(5):535–544
Harris FM, Tesseur I, Brecht WJ, Xu Q, Mullendorff K, Chang S, Wyss-Coray T, Mahley RW et al (2004) Astroglial regulation of apolipoprotein E expression in neuronal cells. Implications for Alzheimer’s disease. J Biol Chem 279(5):3862–3868. doi:10.1074/jbc.M309475200
Aoki K, Uchihara T, Sanjo N, Nakamura A, Ikeda K, Tsuchiya K, Wakayama Y (2003) Increased expression of neuronal apolipoprotein E in human brain with cerebral infarction. Stroke 34(4):875–880. doi:10.1161/01.STR.0000064320.73388.C6
Xu Q, Bernardo A, Walker D, Kanegawa T, Mahley RW, Huang Y (2006) Profile and regulation of apolipoprotein E (ApoE) expression in the CNS in mice with targeting of green fluorescent protein gene to the ApoE locus. J Neurosci 26(19):4985–4994. doi:10.1523/JNEUROSCI.5476-05.2006
Raffaï RL, Dong L-M, Farese RV, Weisgraber KH (2001) Introduction of human apolipoprotein E4 “domain interaction” into mouse apolipoprotein E. Proc Natl Acad Sci U S A 98(20):11587–11591. doi:10.1073/pnas.201279298
Weisgraber KH (1994) Apolipoprotein E: structure-function relationships. Adv Protein Chem 45:249–302
Dong L-M, Weisgraber KH (1996) Human apolipoprotein E4 domain interaction: arginine 61 and glutamic acid 255 interact to direct the preference for very low density lipoproteins. J Biol Chem 271(32):19053–19057. doi:10.1074/jbc.271.32.19053
Harris FM, Brecht WJ, Xu Q, Tesseur I, Kekonius L, Wyss-Coray T, Fish JD, Masliah E et al (2003) Carboxyl-terminal-truncated apolipoprotein E4 causes Alzheimer’s disease-like neurodegeneration and behavioral deficits in transgenic mice. Proc Natl Acad Sci U S A 100(19):10966–10971. doi:10.1073/pnas.1434398100
Rohn TT, Catlin LW, Coonse KG, Habig JW (2012) Identification of an amino-terminal fragment of apolipoprotein E4 that localizes to neurofibrillary tangles of the Alzheimer’s disease brain. Brain Res 1475:106–115. doi:10.1016/j.brainres.2012.08.003
Huang Y, Liu XQ, Wyss-Coray T, Brecht WJ, Sanan DA, Mahley RW (2001) Apolipoprotein E fragments present in Alzheimer’s disease brains induce neurofibrillary tangle-like intracellular inclusions in neurons. Proc Natl Acad Sci U S A 98(15):8838–8843. doi:10.1073/pnas.151254698
Ljungberg MC, Dayanandan R, Asuni A, Rupniak TH, Anderton BH, Lovestone S (2002) Truncated apoE forms tangle-like structures in a neuronal cell line. Neuroreport 13(6):867–870
Radde R, Bolmont T, Kaeser SA, Coomaraswamy J, Lindau D, Stoltze L, Calhoun ME, Jaggi F et al (2006) Abeta42-driven cerebral amyloidosis in transgenic mice reveals early and robust pathology. EMBO Rep 7(9):940–946. doi:10.1038/sj.embor.7400784
Breyhan H, Wirths O, Duan K, Marcello A, Rettig J, Bayer TA (2009) APP/PS1KI bigenic mice develop early synaptic deficits and hippocampus atrophy. Acta Neuropathol 117(6):677–685. doi:10.1007/s00401-009-0539-7
Brecht WJ, Harris FM, Chang S, Tesseur I, Yu GQ, Xu Q, Dee Fish J, Wyss-Coray T et al (2004) Neuron-specific apolipoprotein e4 proteolysis is associated with increased tau phosphorylation in brains of transgenic mice. J Neurosci 24(10):2527–2534
Elliott DA, Tsoi K, Holinkova S, Chan SL, Kim WS, Halliday GM, Rye KA, Garner B (2011) Isoform-specific proteolysis of apolipoprotein-E in the brain. Neurobiol Aging 32(2):257–271. doi:10.1016/j.neurobiolaging.2009.02.006
Bien-Ly N, Andrews-Zwilling Y, Xu Q, Bernardo A, Wang C, Huang Y (2011) C-terminal-truncated apolipoprotein (apo) E4 inefficiently clears amyloid-beta (Abeta) and acts in concert with Abeta to elicit neuronal and behavioral deficits in mice. Proc Natl Acad Sci U S A 108(10):4236–4241. doi:10.1073/pnas.1018381108
Jones PB, Adams KW, Rozkalne A, Spires-Jones TL, Hshieh TT, Hashimoto T, von Armin CAF, Mielke M et al (2011) Apolipoprotein E: isoform specific differences in tertiary structure and interaction with amyloid-β in human Alzheimer brain. PLoS One 6(1):e14586
Wellnitz S, Friedlein A, Bonanni C, Anquez V, Goepfert F, Loetscher H, Adessi C, Czech C (2005) A 13 kDa carboxy-terminal fragment of ApoE stabilizes Abeta hexamers. J Neurochem 94(5):1351–1360. doi:10.1111/j.1471-4159.2005.03295.x
Huang Y, Weisgraber KH, Mucke L, Mahley RW (2004) Apolipoprotein E: diversity of cellular origins, structural and biophysical properties, and effects in Alzheimer’s disease. J Mol Neurosci 23(3):189–204. doi:10.1385/jmn:23:3:189
Mahley RW, Weisgraber KH, Huang Y (2006) Apolipoprotein E4: a causative factor and therapeutic target in neuropathology, including Alzheimer’s disease. Proc Natl Acad Sci U S A 103(15):5644–5651. doi:10.1073/pnas.0600549103
Weisgraber KH, Rall SC Jr, Mahley RW (1981) Human E apoprotein heterogeneity. Cysteine-arginine interchanges in the amino acid sequence of the apo-E isoforms. J Biol Chem 256(17):9077–9083
Weisgraber KH (1990) Apolipoprotein E distribution among human plasma lipoproteins: role of the cysteine-arginine interchange at residue 112. J Lipid Res 31(8):1503–1511
Morrow JA, Hatters DM, Lu B, Höchtl P, Oberg KA, Rupp B, Weisgraber KH (2002) Apolipoprotein E4 forms a molten globule: a potential basis for its association with disease. J Biol Chem 277(52):50380–50385. doi:10.1074/jbc.M204898200
Bales KR, Liu F, Wu S, Lin S, Koger D, DeLong C, Hansen JC, Sullivan PM et al (2009) Human APOE isoform-dependent effects on brain beta-amyloid levels in PDAPP transgenic mice. J Neurosci 29(21):6771–6779. doi:10.1523/JNEUROSCI.0887-09.2009
Holtzman DM, Bales KR, Tenkova T, Fagan AM, Parsadanian M, Sartorius LJ, Mackey B, Olney J et al (2000) Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A 97(6):2892–2897
Verghese PB, Castellano JM, Garai K, Wang Y, Jiang H, Shah A, Bu G, Frieden C et al (2013) ApoE influences amyloid-beta (Abeta) clearance despite minimal apoE/Abeta association in physiological conditions. Proc Natl Acad Sci U S A 110(19):E1807–E1816. doi:10.1073/pnas.1220484110
Christensen DZ, Schneider-Axmann T, Lucassen PJ, Bayer TA, Wirths O (2010) Accumulation of intraneuronal Abeta correlates with ApoE4 genotype. Acta Neuropathol 119:555–566. doi:10.1007/s00401-010-0666-1
Bales KR, Verina T, Cummins DJ, Du Y, Dodel RC, Saura J, Fishman CE, DeLong CA et al (1999) Apolipoprotein E is essential for amyloid deposition in the APP(V717F) transgenic mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A 96(26):15233–15238
Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD (1993) Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A 90(5):1977–1981
Bouter Y, Kacprowski T, Weissmann R, Dietrich K, Borgers H, Brauss A, Sperling C, Wirths O et al (2014) Deciphering the molecular profile of plaques, memory decline and neuron loss in two mouse models for Alzheimer’s disease by deep sequencing. Front Aging Neurosci 6:75. doi:10.3389/fnagi.2014.00075
Wirths O, Breyhan H, Marcello A, Cotel MC, Bruck W, Bayer TA (2010) Inflammatory changes are tightly associated with neurodegeneration in the brain and spinal cord of the APP/PS1KI mouse model of Alzheimer’s disease. Neurobiol Aging 31(5):747–757. doi:10.1016/j.neurobiolaging.2008.06.011
Pleasure SJ, Lee VM (1993) NTera 2 cells: a human cell line which displays characteristics expected of a human committed neuronal progenitor cell. J Neurosci Res 35(6):585–602. doi:10.1002/jnr.490350603
Soulie C, Mitchell V, Dupont-Wallois L, Chartier-Harlin MC, Beauvillain JC, Delacourte A, Caillet-Boudin ML (1999) Synthesis of apolipoprotein E (ApoE) mRNA by human neuronal-type SK N SH-SY 5Y cells and its regulation by nerve growth factor and ApoE. Neurosci Lett 265(2):147–150. doi:10.1016/S0304-3940(99)00167-6
Schaffer S, Lam VYM, Ernst IMA, Huebbe P, Rimbach G, Halliwell B (2014) Variability in APOE genotype status in human-derived cell lines: a cause for concern in cell culture studies? Genes & Nutrition 9(1):364. doi:10.1007/s12263-013-0364-4
Bu G (2009) Apolipoprotein E and its receptors in Alzheimer’s disease: pathways, pathogenesis and therapy. Nat Rev Neurosci 10(5):333–344. doi:10.1038/nrn2620
Trommer BL, Shah C, Yun SH, Gamkrelidze G, Pasternak ES, Ye GL, Sotak M, Sullivan PM et al (2004) ApoE isoform affects LTP in human targeted replacement mice. Neuroreport 15(17):2655–2658
Arold S, Sullivan P, Bilousova T, Teng E, Miller CA, Poon WW, Vinters HV, Cornwell LB et al (2012) Apolipoprotein E level and cholesterol are associated with reduced synaptic amyloid beta in Alzheimer’s disease and apoE TR mouse cortex. Acta Neuropathol 123(1):39–52. doi:10.1007/s00401-011-0892-1
Nakamura T, Watanabe A, Fujino T, Hosono T, Michikawa M (2009) Apolipoprotein E4 (1–272) fragment is associated with mitochondrial proteins and affects mitochondrial function in neuronal cells. Mol Neurodegener 4:35. doi:10.1186/1750-1326-4-35
Chang S, Ma T, Miranda RD, Balestra ME, Mahley RW, Huang Y (2005) Lipid- and receptor-binding regions of apolipoprotein E4 fragments act in concert to cause mitochondrial dysfunction and neurotoxicity. Proc Natl Acad Sci U S A 102(51):18694–18699. doi:10.1073/pnas.0508254102
Zhou W, Scott SA, Shelton SB, Crutcher KA (2006) Cathepsin D-mediated proteolysis of apolipoprotein E: possible role in Alzheimer’s disease. Neuroscience 143(3):689–701. doi:10.1016/j.neuroscience.2006.08.019
Amritraj A, Hawkes C, Phinney AL, Mount HT, Scott CD, Westaway D, Kar S (2009) Altered levels and distribution of IGF-II/M6P receptor and lysosomal enzymes in mutant APP and APP + PS1 transgenic mouse brains. Neurobiol Aging 30(1):54–70. doi:10.1016/j.neurobiolaging.2007.05.004
Snir JA, Suchy M, Lawrence KS, Hudson RH, Pasternak SH, Bartha R (2015) Prolonged in vivo retention of a cathepsin D targeted optical contrast agent in a mouse model of Alzheimer’s disease. J Alzheimers Dis 48(1):73–87. doi:10.3233/jad-150123
Casas C, Sergeant N, Itier JM, Blanchard V, Wirths O, van der Kolk N, Vingtdeux V, van de Steeg E et al (2004) Massive CA1/2 neuronal loss with intraneuronal and N-terminal truncated Abeta42 accumulation in a novel Alzheimer transgenic model. Am J Pathol 165(4):1289–1300. doi:10.1016/S0002-9440(10)63388-3
Bayer TA, Wirths O (2008) Review on the APP/PS1KI mouse model: intraneuronal Abeta accumulation triggers axonopathy, neuron loss and working memory impairment. Genes Brain Behav 7(Suppl 1):6–11. doi:10.1111/j.1601-183X.2007.00372.x
Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, Guillozet-Bongaarts A, Ohno M et al (2006) Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. J Neurosci 26(40):10129–10140
Jawhar S, Trawicka A, Jenneckens C, Bayer TA, Wirths O (2012) Motor deficits, neuron loss, and reduced anxiety coinciding with axonal degeneration and intraneuronal Abeta aggregation in the 5XFAD mouse model of Alzheimer’s disease. Neurobiol Aging 33(1):196.e129–196.e140. doi:10.1016/j.neurobiolaging.2010.05.027
Lichtenthaler SF, Ida N, Multhaup G, Masters CL, Beyreuther K (1997) Mutations in the transmembrane domain of APP altering gamma-secretase specificity. Biochemistry 36(49):15396–15403
Ferreira S, Dupire M-J, Delacourte A, Najib J, Caillet-Boudin M-L (2000) Synthesis and regulation of apolipoprotein E during the differentiation of human neuronal precursor NT2/D1 cells into postmitotic neurons. Exp Neurol 166(2):415–421. doi:10.1006/exnr.2000.7510
Hahn S, Bruning T, Ness J, Czirr E, Baches S, Gijsen H, Korth C, Pietrzik CU et al (2011) Presenilin-1 but not amyloid precursor protein mutations present in mouse models of Alzheimer’s disease attenuate the response of cultured cells to gamma-secretase modulators regardless of their potency and structure. J Neurochem 116(3):385–395. doi:10.1111/j.1471-4159.2010.07118.x
Acknowledgments
This work was supported by the Alzheimer Forschung Initiative e.V. (to O.W., grant #12802).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures were performed according to German guidelines for animal care and approved by the University of Goettingen Medical Center Institutional Animal Care and Use Committee.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
Supplemental Fig. 1. ApoE fragmentation in APPPS1-21 mice. Supplemental Fig. 2. ApoE fragments accumulate in synaptosomes of APP/PS1KI mice. (PDF 306 kb)
Rights and permissions
About this article
Cite this article
Saul, A., Wirths, O. Endogenous Apolipoprotein E (ApoE) Fragmentation Is Linked to Amyloid Pathology in Transgenic Mouse Models of Alzheimer’s Disease. Mol Neurobiol 54, 319–327 (2017). https://doi.org/10.1007/s12035-015-9674-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9674-4