Abstract
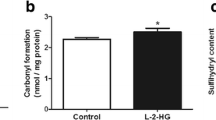
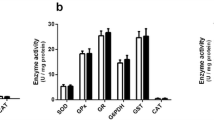
Accumulation of glycine (GLY) is the biochemical hallmark of glycine encephalopathy (GE), an aminoacidopathy characterized by severe neurological dysfunction that may lead to early death. In the present study, we evaluated the effect of a single intracerebroventricular administration of GLY on bioenergetics, redox homeostasis, and histopathology in brain of neonatal rats. Our results demonstrated that GLY decreased the activities of the respiratory chain complex IV and creatine kinase, induced reactive species generation, and diminished glutathione (GSH) levels 1, 5, and 10 days after GLY injection in cerebral cortex of 1-day-old rats. GLY also increased malondialdehyde (MDA) levels 5 days after GLY infusion in this brain region. Furthermore, GLY differentially modulated the activities of superoxide dismutase, catalase, and glutathione peroxidase depending on the period tested after GLY administration. In contrast, bioenergetics and redox parameters were not altered in brain of 5-day-old rats. Regarding the histopathological analysis, GLY increased S100β staining in cerebral cortex and striatum, and GFAP in corpus callosum of 1-day-old rats 5 days after injection. Finally, we verified that melatonin prevented the decrease of complex IV and CK activities and GSH concentrations, and the increase of MDA levels and S100β staining caused by GLY. Based on our findings, it may be presumed that impairment of redox and energy homeostasis and glial reactivity induced by GLY may contribute to the neurological dysfunction observed in GE.










Similar content being viewed by others
References
Hamosh A, Johnston MV (2001) Non-ketotic hyperglycinemia. In: Scriver CR, Beaudet A, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited disease, vol Editors, 8th edn. McGraw-Hill, New York, pp 2065–2078
Applegarth DA, Toone JR, Lowry RB (2000) Incidence of inborn errors of metabolism in British Columbia, 1969–1996. Pediatrics 105(1):e10
Heindel W, Kugel H, Roth B (1993) Noninvasive detection of increased glycine content by proton MR spectroscopy in the brains of two infants with nonketotic hyperglycinemia. Am J Neuroradiol 14(3):629–635
Raghavendra S, Ashalatha R, Thomas SV, Kesavadas C (2007) Focal neuronal loss, reversible subcortical focal T2 hypointensity in seizures with a nonketotic hyperglycemic hyperosmolar state. Neuroradiology 49(4):299–305. doi:10.1007/s00234-006-0189-6
Shuman RM, Leech RW, Scott CR (1978) The neuropathology of the nonketotic and ketotic hyperglycinemias: three cases. Neurology 28(2):139–146
Bekiesiniska-Figatowska M, Rokicki D, Walecki J (2001) MRI in nonketotic hyperglycinaemia: case report. Neuroradiology 43(9):792–793
Hennermann JB, Berger JM, Grieben U, Scharer G, Van Hove JL (2012) Prediction of long-term outcome in glycine encephalopathy: a clinical survey. J Inherit Metab Dis 35(2):253–261. doi:10.1007/s10545-011-9398-1
Tsuyusaki Y, Shimbo H, Wada T, Iai M, Tsuji M, Yamashita S, Aida N, Kure S, Osaka H (2012) Paradoxical increase in seizure frequency with valproate in nonketotic hyperglycinemia. Brain Dev 34(1):72–75. doi:10.1016/j.braindev.2011.01.005
Hara H, Sukamoto T, Kogure K (1993) Mechanism and pathogenesis of ischemia-induced neuronal damage. Prog Neurobiol 40(6):645–670. doi:10.1016/0301-0082(93)90009-H
Kure S, Tada K, Narisawa K (1997) Nonketotic hyperglycinemia: biochemical, molecular, and neurological aspects. Jpn J Hum Genet 42(1):13–22. doi:10.1007/BF02766917
Applegarth DA, Toone JR (2001) Nonketotic hyperglycinemia (glycine encephalopathy): laboratory diagnosis. Mol Genet Metab 74(1–2):139–146. doi:10.1006/mgme.2001.3224
Kono Y, Shigetomi E, Inoue K, Kato F (2007) Facilitation of spontaneous glycine release by anoxia potentiates NMDA receptor current in the hypoglossal motor neurons of the rat. Eur J Neurosci 25(6):1748–1756. doi:10.1111/j.1460-9568.2007.05426.x
Katsuki H, Watanabe Y, Fujimoto S, Kume T, Akaike A (2007) Contribution of endogenous glycine and d-serine to excitotoxic and ischemic cell death in rat cerebrocortical slice cultures. Life Sci 81(9):740–749. doi:10.1016/j.lfs.2007.07.001
Leipnitz G, Solano AF, Seminotti B, Amaral AU, Fernandes CG, Beskow AP, Dutra Filho CS, Wajner M (2009) Glycine provokes lipid oxidative damage and reduces the antioxidant defenses in brain cortex of young rats. Cell Mol Neurobiol 29(2):253–261. doi:10.1007/s10571-008-9318-6
Busanello EN, Moura AP, Viegas CM, Zanatta A, da Costa FG, Schuck PF, Wajner M (2010) Neurochemical evidence that glycine induces bioenergetical dysfunction. Neurochem Int 56(8):948–954. doi:10.1016/j.neuint.2010.04.002
Seminotti B, Knebel LA, Fernandes CG, Amaral AU, da Rosa MS, Eichler P, Leipnitz G, Wajner M (2011) Glycine intrastriatal administration induces lipid and protein oxidative damage and alters the enzymatic antioxidant defenses in rat brain. Life Sci 89(7–8):276–281. doi:10.1016/j.lfs.2011.06.013
Pai YJ, Leung KY, Savery D, Hutchin T, Prunty H, Heales S, Brosnan ME, Brosnan JT, Copp AJ, Greene ND (2015) Glycine decarboxylase deficiency causes neural tube defects and features of non-ketotic hyperglycinemia in mice. Nat Commun 6:6388. doi:10.1038/ncomms7388
Olivera-Bravo S, Fernandez A, Sarlabos MN, Rosillo JC, Casanova G, Jimenez M, Barbeito L (2011) Neonatal astrocyte damage is sufficient to trigger progressive striatal degeneration in a rat model of glutaric acidemia-I. PLoS One 6(6):e20831. doi:10.1371/journal.pone.0020831
Olivera-Bravo S, Isasi E, Fernandez A, Rosillo JC, Jimenez M, Casanova G, Sarlabos MN, Barbeito L (2014) White matter injury induced by perinatal exposure to glutaric acid. Neurotox Res 25(4):381–391. doi:10.1007/s12640-013-9445-9
Olivier P, Fontaine RH, Loron G, Van Steenwinckel J, Biran V, Massonneau V, Kaindl A, Dalous J, Charriaut-Marlangue C, Aigrot MS, Pansiot J, Verney C, Gressens P, Baud O (2009) Melatonin promotes oligodendroglial maturation of injured white matter in neonatal rats. PLoS One 4(9):e7128. doi:10.1371/journal.pone.0007128
Evelson P, Travacio M, Repetto M, Escobar J, Llesuy S, Lissi EA (2001) Evaluation of total reactive antioxidant potential (TRAP) of tissue homogenates and their cytosols. Arch Biochem Biophys 388(2):261–266. doi:10.1006/abbi.2001.2292
Schapira AHV, Mann VM, Cooper JM, Dexter D, Daniel SE, Jenner P, Clark JB, Marsden CD (1990) Anatomic and disease specificity of NADH CoQ1 reductase (complex I) deficiency in Parkinson’s disease. J Neurochem 55(6):2142–2145
Fischer JC, Ruitenbeek W, Berden JA, Trijbels JM, Veerkamp JH, Stadhouders AM, Sengers RC, Janssen AJ (1985) Differential investigation of the capacity of succinate oxidation in human skeletal muscle. Clin Chim Acta 153(1):23–36
Rustin P, Chretien D, Bourgeron T, Gerard B, Rotig A, Saudubray JM, Munnich A (1994) Biochemical and molecular investigations in respiratory chain deficiencies. Clin Chim Acta 228(1):35–51
da Silva CG, Ribeiro CAJ, Leipnitz G, Dutra CS, Wyse ATS, Wannmacher CMD, Sarkis JJF, Jakobs C, Wajner M (2002) Inhibition of cytochrome c oxidase activity in rat cerebral cortex and human skeletal muscle by d-2-hydroxyglutaric acid in vitro. Biochim Biophys Acta 1586(1):81–91. doi:10.1016/S09254439(01)00088-6
Hughes BP (1962) A method for estimation of serum creatine kinase and its use in comparing creatine kinase and aldolase activity in normal and pathological sera. Clin Chim Acta 7(5):597–603
da Silva CG, Bueno ARF, Schuck PF, Leipnitz G, Ribeiro CAJ, Rosa RB, Dutra CS, Wyse ATS, Wannmacher CMD, Wajner M (2004) Inhibition of creatine kinase activity from rat cerebral cortex by d-2-hydroxyglutaric acid in vitro. Neurochem Int 44(1):45–52. doi:10.1016/S0197-0186(03)00098-6
Yagi K (1998) Simple procedure for specific assay of lipid hydroperoxides in serum or plasma. Methods Mol Biol 108:107–110. doi:10.1385/0-89603-472-0:107
LeBel CP, Ischiropoulos H, Bondy SC (1992) Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol 5(2):227–231
Browne RW, Armstrong D (1998) Reduced glutathione and glutathione disulfide. Methods Mol Biol 108:347–352. doi:10.1385/0-89603-472-0:347
Marklund SL (1985) Product of extracellular-superoxide dismutase catalysis. FEBS Lett 184(2):237–239
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Wendel A (1981) Glutathione peroxidase. Methods Enzymol 77:325–333
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65(1–2):55–63
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Brun A, Borjeson M, Hultberg B, Sjoblad S, Akesson H, Litwin E (1979) Neonatal non-ketotic hyperglycinemia: a clinical, biochemical and neuropathological study including electronmicroscopic findings. Neuropadiatrie 10(2):195–205. doi:10.1055/s-0028-1085325
Korman SH, Wexler ID, Gutman A, Rolland MO, Kanno J, Kure S (2006) Treatment from birth of nonketotic hyperglycinemia due to a novel GLDC mutation. Ann Neurol 59(2):411–415. doi:10.1002/ana.20759
Halliwell B, Gutteridge JMC (2007) Measurement of reactive species. Free radicals in biology and medicine, 4th edn. Oxford University Press, Oxford
Moura AP, Grings M, Marcowich GF, Bumbel AP, Parmeggiani B, de Moura Alvorcem L, Wajner M, Leipnitz G (2014) Evidence that glycine induces lipid peroxidation and decreases glutathione concentrations in rat cerebellum. Mol Cell Biochem 395(1–2):125–134. doi:10.1007/s11010-014-2118-z
Reiter RJ, Tan DX, Rosales-Corral S, Manchester LC (2013) The universal nature, unequal distribution and antioxidant functions of melatonin and its derivatives. Mini Rev Med Chem 13(3):373–384. doi:10.2174/1389557511313030006
Bromme HJ, Morke W, Peschke D, Ebelt H (2000) Scavenging effect of melatonin on hydroxyl radicals generated by alloxan. J Pineal Res 29(4):201–208. doi:10.1034/j.1600-0633.2002.290402.x
Matuszak Z, Reszka K, Chignell CF (1997) Reaction of melatonin and related indoles with hydroxyl radicals: EPR and spin trapping investigations. Free Radic Biol Med 23(3):367–372. doi:10.1016/S0891-5849(96)00614-4
Stasica P, Ulanski P, Rosiak JM (1998) Melatonin as a hydroxyl radical scavenger. J Pineal Res 25(1):65–66
Reyes-Toso CF, Rebagliati IR, Ricci CR, Linares LM, Albornoz LE, Cardinali DP, Zaninovich A (2006) Effect of melatonin treatment on oxygen consumption by rat liver mitochondria. Amino Acids 31(3):299–302. doi:10.1007/s00726-005-0280-z
Garcia JJ, Lopez-Pingarron L, Almeida-Souza P, Tres A, Escudero P, Garcia-Gil FA, Tan DX, Reiter RJ, Ramirez JM, Bernal-Perez M (2014) Protective effects of melatonin in reducing oxidative stress and in preserving the fluidity of biological membranes: a review. J Pineal Res 56(3):225–237. doi:10.1111/jpi.12128
Martin M, Macias M, Escames G, Reiter RJ, Agapito MT, Ortiz GG, Acuna-Castroviejo D (2000) Melatonin-induced increased activity of the respiratory chain complexes I and IV can prevent mitochondrial damage induced by ruthenium red in vivo. J Pineal Res 28(4):242–248. doi:10.1034/j.1600-079X.2000.280407.x
Gan L, Johnson JA (2014) Oxidative damage and the Nrf2-ARE pathway in neurodegenerative diseases. Biochim Biophys Acta 1842(8):1208–1218. doi:10.1016/j.bbadis.2013.12.011
Itoh K, Ishii T, Wakabayashi N, Yamamoto M (1999) Regulatory mechanisms of cellular response to oxidative stress. Free Radic Res 31(4):319–324
Johnson JA, Johnson DA, Kraft AD, Calkins MJ, Jakel RJ, Vargas MR, Chen PC (2008) The Nrf2-ARE pathway: an indicator and modulator of oxidative stress in neurodegeneration. Ann N Y Acad Sci 1147:61–69. doi:10.1196/annals.1427.036
Shih AY, Johnson DA, Wong G, Kraft AD, Jiang L, Erb H, Johnson JA, Murphy TH (2003) Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. J Neurosci 23(8):3394–3406
Limon-Pacheco JH, Gonsebatt ME (2010) The glutathione system and its regulation by neurohormone melatonin in the central nervous system. Cent Nerv Syst Agents Med Chem 10(4):287–297. doi:10.2174/187152410793429683
Kaushik S, Kaur J (2003) Chronic cold exposure affects the antioxidant defense system in various rat tissues. Clin Chim Acta 333(1):69–77. doi:10.1016/S0009-8981(03)00171-2
Singh P, Jain A, Kaur G (2004) Impact of hypoglycemia and diabetes on CNS: correlation of mitochondrial oxidative stress with DNA damage. Mol Cell Biochem 260(1–2):153–159
Jafari M (2007) Dose- and time-dependent effects of sulfur mustard on antioxidant system in liver and brain of rat. Toxicology 231(1):30–39. doi:10.1016/j.tox.2006.11.048
Costa MZ, da Silva TM, Flores NP, Schmitz F, da Silva Scherer EB, Viau CM, Saffi J, Barschak AG, de Souza Wyse AT, Spanevello RM, Stefanello FM (2013) Methionine and methionine sulfoxide alter parameters of oxidative stress in the liver of young rats: in vitro and in vivo studies. Mol Cell Biochem 384(1–2):21–28. doi:10.1007/s11010-013-1777-5
da Rosa MS, Seminotti B, Amaral AU, Fernandes CG, Gasparotto J, Moreira JC, Gelain DP, Wajner M, Leipnitz G (2013) Redox homeostasis is compromised in vivo by the metabolites accumulating in 3-hydroxy-3-methylglutaryl-CoA lyase deficiency in rat cerebral cortex and liver. Free Radic Res 47(12):1066–1075. doi:10.3109/10715762.2013.853876
Sasso S, Dalmedico L, Delwing-Dal Magro D, Wyse AT, Delwing-de Lima D (2014) Effect of N-acetylarginine, a metabolite accumulated in hyperargininemia, on parameters of oxidative stress in rats: protective role of vitamins and l-NAME. Cell Biochem Funct 32(6):511–519. doi:10.1002/cbf.3045
Viegas CM, Zanatta A, Grings M, Hickmann FH, Monteiro WO, Soares LE, Sitta A, Leipnitz G, de Oliveira FH, Wajner M (2014) Disruption of redox homeostasis and brain damage caused in vivo by methylmalonic acid and ammonia in cerebral cortex and striatum of developing rats. Free Radic Res 48(6):659–669. doi:10.3109/10715762.2014.898842
Ferriero DM, Miller SP (2010) Imaging selective vulnerability in the developing nervous system. J Anat 217(4):429–435. doi:10.1111/j.1469-7580.2010.01226.x
Ikonomidou C, Kaindl AM (2011) Neuronal death and oxidative stress in the developing brain. Antioxid Redox Signal 14(8):1535–1550. doi:10.1089/ars.2010.3581
Paupe A, Bidat L, Sonigo P, Lenclen R, Molho M, Ville Y (2002) Prenatal diagnosis of hypoplasia of the corpus callosum in association with non-ketotic hyperglycinemia. Ultrasound Obstet Gynecol 20(6):616–619. doi:10.1046/j.1469-0705.2002.00869
Press GA, Barshop BA, Haas RH, Nyhan WL, Glass RF, Hesselink JR (1989) Abnormalities of the brain in nonketotic hyperglycinemia: MR manifestations. AJNR Am J Neuroradiol 10(2):315–321
del Toro M, Arranz JA, Macaya A, Riudor E, Raspall M, Moreno A, Vazquez E, Ortega A, Matsubara Y, Kure S, Roig M (2006) Progressive vacuolating glycine leukoencephalopathy with pulmonary hypertension. Ann Neurol 60(1):148–152. doi:10.1002/ana.20887
Sorci G, Bianchi R, Riuzzi F, Tubaro C, Arcuri C, Giambanco I, Donato R (2010). S100B protein, a damage-associated molecular pattern protein in the brain and heart, and beyond. Cardiovasc Psychiatry Neurol. doi:10.1155/2010/656481
Cerutti SM, Chadi G (2000) S100 immunoreactivity is increased in reactive astrocytes of the visual pathways following a mechanical lesion of the rat occipital cortex. Cell Biol Int 24(1):35–49. doi:10.1006/cbir.1999.0451
Griffin WS, Sheng JG, Mrak RE (1998) Senescence-accelerated overexpression of S100beta in brain of SAMP6 mice. Neurobiol Aging 19(1):71–76
Celikbilek A, Akyol L, Sabah S, Tanik N, Adam M, Celikbilek M, Korkmaz M, Yilmaz N (2014) S100B as a glial cell marker in diabetic peripheral neuropathy. Neurosci Lett 558:53–57. doi:10.1016/j.neulet.2013.10.067
Avishai-Eliner S, Brunson KL, Sandman CA, Baram TZ (2002) Stressed-out, or in (utero)? Trends Neurosci 25(10):518–524
Rice D, Barone S Jr (2000) Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect 108(Suppl 3):511–533
Acknowledgments
The authors declare that there is no conflict of interest. This work was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Programa de Apoio a Núcleos de Excelência (PRONEX II), Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS), Pró-Reitoria de Pesquisa/Universidade Federal do Rio Grande do Sul (PROPESQ/UFRGS), Financiadora de estudos e projetos (FINEP), Rede Instituto Brasileiro de Neurociência (IBN-Net) no. 01.06.0842-00, and Instituto Nacional de Ciência e Tecnologia em Excitotoxicidade e Neuroproteção (INCT-EN).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moura, A.P., Parmeggiani, B., Grings, M. et al. Intracerebral Glycine Administration Impairs Energy and Redox Homeostasis and Induces Glial Reactivity in Cerebral Cortex of Newborn Rats. Mol Neurobiol 53, 5864–5875 (2016). https://doi.org/10.1007/s12035-015-9493-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9493-7