Abstract
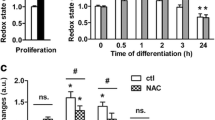
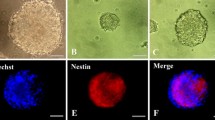
Naphthoquinones are bioactive compounds widespread in nature that impact on several cellular pathways, including cell proliferation and survival, by acting as prooxidants and electrophiles. We have previously described the role of the synthetic isoxazole condensed 1,4-naphthoquinone derivative 1a in preventing apoptosis induced by distinct stimuli in several cell models. In addition, apoptosis regulators and executioners may control neural stem cell (NSC) fate, without involving cell death per se. Here, we hypothesize that 1a might also play a role in NSC fate decision. We found that exposure to 1a shifts NSC differentiation potential from neurogenic to gliogenic lineage and involves the generation of reactive oxygen species, without increasing cell death. Modulation of caspases and calpains, using cysteine protease inhibitors, failed to mimic 1a effects. In addition, incubation with the naphthoquinone derivative resulted in upregulation and nuclear translocation of antioxidant responsive proteins, Nrf2 and Sirt1, which in turn may mediate 1a-directed shift in NSC differentiation. In fact, antioxidants halted the shift in NSC differentiation potential from neurogenic to gliogenic lineage, while strongly reducing reactive oxygen species generation and Nrf2 and Sirt1 nuclear translocation in NSC exposed to 1a. Collectively, these data support a new role for a specific naphthoquinone derivative in NSC fate decision and underline the importance of redox environment control.






Similar content being viewed by others
Abbreviations
- DCF:
-
2′,7′-Dichlorofluorescein
- FBS:
-
Fetal bovine serum
- GFAP:
-
Glial fibrillary acidic protein
- H2DCFDA:
-
2′,7′-Dichlorodihydrofluorescein diacetate
- LA:
-
Lipoic acid
- NAC:
-
N-Acetylcysteine
- Nrf2:
-
Nuclear factor erythroid 2-related factor 2
- NSC:
-
Neural stem cells
- NS-TGFP:
-
Adherent mouse Tau-GFP NSC
- PBS:
-
Phosphate buffer saline
- ROS:
-
Reactive oxygen species
- Sirt1:
-
Sirtuin 1
References
Murry CE, Keller G (2008) Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell 132(4):661–680
Gage FH (2000) Mammalian neural stem cells. Science 287(5457):1433–1438
Daadi MM (2011) Novel paths towards neural cellular products for neurological disorders. Regen Med 6(6 Suppl):25–30
Lyssiotis CA, Lairson LL, Boitano AE, Wurdak H, Zhu S, Schultz PG (2011) Chemical control of stem cell fate and developmental potential. Angew Chem Int Ed Engl 50(1):200–242
Kumagai Y, Shinkai Y, Miura T, Cho AK (2012) The chemical biology of naphthoquinones and its environmental implications. Annu Rev Pharmacol Toxicol 52:221–247
Bolton JL, Trush MA, Penning TM, Dryhurst G, Monks TJ (2000) Role of quinones in toxicology. Chem Res Toxicol 13(3):135–160
Ray PD, Huang BW, Tsuji Y (2012) Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 24(5):981–990
Baird L, Dinkova-Kostova AT (2011) The cytoprotective role of the Keap1–Nrf2 pathway. Arch Toxicol 85(4):241–272
Rajendran R, Garva R, Krstic-Demonacos M, Demonacos C (2011) Sirtuins: molecular traffic lights in the crossroad of oxidative stress, chromatin remodeling, and transcription. J Biomed Biotechnol 2011:368276
Santos DM, Santos MM, Viana RJ, Castro RE, Moreira R, Rodrigues CM (2009) Naphtho[2,3-d]isoxazole-4,9-dione-3-carboxylates: potent, non-cytotoxic, antiapoptotic agents. Chem Biol Interact 180(2):175–182
Castro RE, Santos MM, Gloria PM, Ribeiro CJ, Ferreira DM, Xavier JM, Moreira R, Rodrigues CM (2010) Cell death targets and potential modulators in Alzheimer's disease. Curr Pharm Des 16(25):2851–2864
Fernando P, Kelly JF, Balazsi K, Slack RS, Megeney LA (2002) Caspase 3 activity is required for skeletal muscle differentiation. Proc Natl Acad Sci U S A 99(17):11025–11030
Fernando P, Brunette S, Megeney LA (2005) Neural stem cell differentiation is dependent upon endogenous caspase 3 activity. FASEB J 19(12):1671–1673
Fernando P, Megeney LA (2007) Is caspase-dependent apoptosis only cell differentiation taken to the extreme? FASEB J 21(1):8–17
Stiewe T (2007) The p53 family in differentiation and tumorigenesis. Nat Rev Cancer 7(3):165–168
Aranha MM, Sola S, Low WC, Steer CJ, Rodrigues CM (2009) Caspases and p53 modulate FOXO3A/Id1 signaling during mouse neural stem cell differentiation. J Cell Biochem 107(4):748–758
Santos DM, Xavier JM, Morgado AL, Sola S, Rodrigues CM (2012) Distinct regulatory functions of calpain 1 and 2 during neural stem cell self-renewal and differentiation. PLoS One 7(3):e33468
Aranha MM, Santos DM, Xavier JM, Low WC, Steer CJ, Sola S, Rodrigues CM (2010) Apoptosis-associated microRNAs are modulated in mouse, rat and human neural differentiation. BMC Genomics 11:514
Aranha MM, Santos DM, Sola S, Steer CJ, Rodrigues CM (2011) miR-34a regulates mouse neural stem cell differentiation. PLoS One 6(8):e21396.
Sola S, Aranha MM, Rodrigues CM (2012) Driving apoptosis-relevant proteins toward neural differentiation. Mol Neurobiol. doi:10.1007/s12035-012-8289-2
Jones DP (2008) Radical-free biology of oxidative stress. Am J Physiol Cell Physiol 295(4):C849–C868
Vieira HL, Alves PM, Vercelli A (2011) Modulation of neuronal stem cell differentiation by hypoxia and reactive oxygen species. Prog Neurobiol 93(3):444–455
Wang W, Esbensen Y, Kunke D, Suganthan R, Rachek L, Bjoras M, Eide L (2011) Mitochondrial DNA damage level determines neural stem cell differentiation fate. J Neurosci 31(26):9746–9751
Wang W, Osenbroch P, Skinnes R, Esbensen Y, Bjoras M, Eide L (2010) Mitochondrial DNA integrity is essential for mitochondrial maturation during differentiation of neural stem cells. Stem Cells 28(12):2195–2204
Luo Y, Mughal MR, Ouyang TG, Jiang H, Luo W, Yu QS, Greig NH, Mattson MP (2010) Plumbagin promotes the generation of astrocytes from rat spinal cord neural progenitors via activation of the transcription factor Stat3. J Neurochem 115(6):1337–1349
Lametsch R, Lonergan S, Huff-Lonergan E (2008) Disulfide bond within mu-calpain active site inhibits activity and autolysis. Biochim Biophys Acta 1784(9):1215–1221
Carlin KR, Huff-Lonergan E, Rowe LJ, Lonergan SM (2006) Effect of oxidation, pH, and ionic strength on calpastatin inhibition of mu- and m-calpain. J Anim Sci 84(4):925–937
Borutaite V, Brown GC (2001) Caspases are reversibly inactivated by hydrogen peroxide. FEBS Lett 500(3):114–118
Barbouti A, Amorgianiotis C, Kolettas E, Kanavaros P, Galaris D (2007) Hydrogen peroxide inhibits caspase-dependent apoptosis by inactivating procaspase-9 in an iron-dependent manner. Free Radic Biol Med 43(10):1377–1387
de Lucas NC, Correa RJ, Garden SJ, Santos G, Rodrigues R, Carvalho CE, Ferreira SB, Netto-Ferreira JC, Ferreira VF, Miro P, Marin ML, Miranda MA (2012) Singlet oxygen production by pyrano and furano 1,4-naphthoquinones in non-aqueous medium. Photochem Photobiol Sci 11(7):1201–1209
Kobayashi M, Li L, Iwamoto N, Nakajima-Takagi Y, Kaneko H, Nakayama Y, Eguchi M, Wada Y, Kumagai Y, Yamamoto M (2009) The antioxidant defense system Keap1–Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol Cell Biol 29(2):493–502
Surh YJ, Kundu JK, Na HK (2008) Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med 74(13):1526–1539
Bock KW (2012) Ah receptor- and Nrf2-gene battery members: modulators of quinone-mediated oxidative and endoplasmic reticulum stress. Biochem Pharmacol 83(7):833–838
Zhao F, Wu T, Lau A, Jiang T, Huang Z, Wang XJ, Chen W, Wong PK, Zhang DD (2009) Nrf2 promotes neuronal cell differentiation. Free Radic Biol Med 47(6):867–879
Kosaka K, Mimura J, Itoh K, Satoh T, Shimojo Y, Kitajima C, Maruyama A, Yamamoto M, Shirasawa T (2010) Role of Nrf2 and p62/ZIP in the neurite outgrowth by carnosic acid in PC12h cells. J Biochem 147(1):73–81
Houtkooper RH, Pirinen E, Auwerx J (2012) Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol 13(4):225–238
Yu J, Auwerx J (2010) Protein deacetylation by SIRT1: an emerging key post-translational modification in metabolic regulation. Pharmacol Res 62(1):35–41
Hisahara S, Chiba S, Matsumoto H, Tanno M, Yagi H, Shimohama S, Sato M, Horio Y (2008) Histone deacetylase SIRT1 modulates neuronal differentiation by its nuclear translocation. Proc Natl Acad Sci U S A 105(40):15599–15604
Prozorovski T, Schulze-Topphoff U, Glumm R, Baumgart J, Schroter F, Ninnemann O, Siegert E, Bendix I, Brustle O, Nitsch R, Zipp F, Aktas O (2008) Sirt1 contributes critically to the redox-dependent fate of neural progenitors. Nat Cell Biol 10(4):385–394
Le Belle JE, Orozco NM, Paucar AA, Saxe JP, Mottahedeh J, Pyle AD, Wu H, Kornblum HI (2011) Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell 8(1):59–71
Webster MJ, O'Grady J, Kleinman JE, Weickert CS (2005) Glial fibrillary acidic protein mRNA levels in the cingulate cortex of individuals with depression, bipolar disorder and schizophrenia. Neuroscience 133(2):453–461
Williams MR, Hampton T, Pearce RK, Hirsch SR, Ansorge O, Thom M, Maier M (2012) Astrocyte decrease in the subgenual cingulate and callosal genu in schizophrenia. Eur Arch Psychiatry Clin Neurosci. doi:10.1007/s00406-012-0328-5
Silva J, Chambers I, Pollard S, Smith A (2006) Nanog promotes transfer of pluripotency after cell fusion. Nature 441(7096):997–1001
Santos MM, Faria N, Iley J, Coles SJ, Hursthouse MB, Martins ML, Moreira R (2010) Reaction of naphthoquinones with substituted nitromethanes. Facile synthesis and antifungal activity of naphtho[2,3-d]isoxazole-4,9-diones. Bioorg Med Chem Lett 20(1):193–195
Pratt T, Sharp L, Nichols J, Price DJ, Mason JO (2000) Embryonic stem cells and transgenic mice ubiquitously expressing a tau-tagged green fluorescent protein. Dev Biol 228(1):19–28
Conti L, Pollard SM, Gorba T, Reitano E, Toselli M, Biella G, Sun Y, Sanzone S, Ying QL, Cattaneo E, Smith A (2005) Niche-independent symmetrical self-renewal of a mammalian tissue stem cell. PLoS Biol 3(9):e283
Pollard SM, Conti L, Sun Y, Goffredo D, Smith A (2006) Adherent neural stem (NS) cells from fetal and adult forebrain. Cereb Cortex 16(Suppl 1):i112–120
Glaser T, Pollard SM, Smith A, Brustle O (2007) Tripotential differentiation of adherently expandable neural stem (NS) cells. PLoS One 2(3):e298
Spiliotopoulos D, Goffredo D, Conti L, Di Febo F, Biella G, Toselli M, Cattaneo E (2009) An optimized experimental strategy for efficient conversion of embryonic stem (ES)-derived mouse neural stem (NS) cells into a nearly homogeneous mature neuronal population. Neurobiol Dis 34(2):320–331
Acknowledgments
We are grateful to Elsa Abranches and Evguenia Bekman (Instituto de Medicina Molecular, University of Lisbon, Lisbon, Portugal) for skillful technical assistance in establishing NS-TGFP cell line culture conditions. The authors also thank Carlos Ribeiro (iMed.UL, University of Lisbon, Lisbon, Portugal) for providing part of the freshly synthesized 1a. We also thank Maria João Gama, Elsa Rodrigues and Andreia Carvalho iMed.UL, University of Lisbon, Lisbon, Portugal) for technical assistance and reagents for ROS assays and Nrf2 detection. This work was supported by grants PTDC/SAU-NMC/117877/2010 and Pest-OE/SAU/UI4013/2011, and fellowship SFRH/BD/42008/2007 (DMS) from Fundação para a Ciência e a Tecnologia, Portugal.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplemental Fig. 1
Chemical formula of naphtho[2,3-d]isoxazole-4,9-dione-3-carboxylate 1a. (TIFF 5965 kb)
Rights and permissions
About this article
Cite this article
Santos, D.M., Santos, M.M.M., Moreira, R. et al. Synthetic Condensed 1,4-naphthoquinone Derivative Shifts Neural Stem Cell Differentiation by Regulating Redox State. Mol Neurobiol 47, 313–324 (2013). https://doi.org/10.1007/s12035-012-8353-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-012-8353-y