Abstract
Breast cancer (BC) is the most common type of cancer among women and is the second most common cause of cancer-related deaths, following lung cancer. Severe toxicity associated with a long-term use of BC chemo- and radiotherapy makes it essential to look for newer therapeutics. Additionally, molecular heterogeneity at both intratumoral and intertumoral levels among BC subtypes is known to result in a differential response to standard therapeutics. Oncolytic viruses (OVs) have emerged as one of the most promising treatment options for BC. Many preclinical and clinical studies have shown that OVs are effective in treating BC, both as a single therapeutic agent and as a part of combination therapies. Combination therapies involving multimodal therapeutics including OVs are becoming popular as they allow to achieve the synergistic therapeutic effects, while minimizing the associated toxicities. Here, we review the OVs for BC therapy in preclinical studies and in clinical trials, both as a monotherapy and as part of a combination therapy. We also briefly discuss the potential therapeutic targets for BC, as these are likely to be critical for the development of new OVs.
Similar content being viewed by others
Introduction
Breast cancer (BC) remains the second most common cause of cancer-related deaths and a major health hazard among women, with over 40,000 anticipated deaths, in 2016, in the USA [1]. Although the recent progress in early diagnostics has improved the outcome of disease, the overall survival rate during the past two decades remains largely unchanged [1]. Additionally, the overall survival and response to therapeutics differ greatly among patients with metastatic BC due to the biodiversity within BC cell subpopulations and tumor microenvironment (TME). Surgery, radiotherapy and chemotherapy remain a part of standard therapy for BC. However, serious adverse effects, inadequacy of treating a metastatic disease and cognitive impairment associated with a long-term use of toxic chemotherapeutics are some of the major concerns associated with the standard therapeutics for BC [2]. Chemotherapy can possibly be effective in controlling metastasis when patients are treated at an early stage of the disease, but patients with an established metastatic disease remain at loss due to a markedly reduced therapeutic efficacy of chemotherapy. Moreover, accumulation of toxicities associated with multiple chemotherapeutic treatment cycles also remains a concern. The complex nature of BC cell subpopulations and the TME makes it unlikely for any of the current therapeutics to act as an “elixir” for all cases of BC.
Oncolytic viruses (OVs) represent a novel class of cancer therapeutics. Most of these agents are designed using pathogenic viruses that infect humans naturally. Replication competent OVs limit the tumor growth and progression not only through the infection and lysis of cancer cells, but also through induction of an anticancer immune response and disruption of the TME. Recently, we have entered an exciting phase for the development of OVs with the approval of T-vec, a recombinant herpes simplex virus (HSV)-1 for melanoma treatment by the US Food and Drug Administration (FDA) [3]. Adenovirus recombinant, H101 is another OV that has been approved for head and neck cancer therapy in China [4].
In this review, we discuss the current therapeutic options for BC with a focus on OVs, as monotherapy and as a part of combination therapies in preclinical and clinical studies. We also review the important potential therapeutic targets for BC which include oncogenes, tumor suppressor genes, growth/hormone receptors (HRs), metabolic and cell cycle pathways and immune checkpoint regulators. Many of these therapeutic targets have been linked with the disease progression and poor outcome in BC patients and can be helpful in directing the development of new therapeutics. Clinical studies testing OVs in BC patients may serve as guidelines for designing new OVs with enhanced therapeutic efficacy. OVs are now being engineered to express therapeutics like anticancer monoclonal antibodies (mAbs) at the target sites eliminating the need of administration of antibodies. One such example is an oncolytic vaccinia virus (VV) expressing mAbs targeting vascular endothelial growth factor (VEGF) can eliminate the need of infusing anti-VEGF antibodies [5].
Breast cancer heterogeneity
BC as many other cancers represents a group of histologically diverse subtypes and not a single disease. Invasive ductal carcinoma (IDC) is the most common type of BC and has been divided into two major types: a non-special type and special type of BC. At least 17 different histologically distinct subtypes of special type of IDC of breast have been identified (reviewed in [6]). Some of the major histological types within the special type of IDC of breast include lobular, tubular, cribriform, medullary, mucinous and papillary. Each of the BC histological subtype can further be graded, based on its proliferation and the degree of differentiation, which correlates with the aggressiveness [7]. Some of the subtypes of IDC of breast show a mixture of characteristics from different histology types, increasing the complexity of histological classification of BC.
Determination of BC histological type is a routine practice. However, one of the major hurdles has been the prediction of the treatment protocol. High-throughput studies determining a molecular profile of BC cells have revealed a significant degree of heterogeneity among BC subpopulations at a molecular level. On this basis, BC has been classified into at least three major subtypes: luminal, human epidermal growth receptor-2 (HER-2) + and basal-like. Luminal tumors express estrogen and progesterone receptors and can be effectively treated using the hormone-based therapies. Oncogene ERBB2 is overexpressed by HER-2 + subtype and responds well to anti-HER-2 therapies in most of the cases. The basal-like subtype is also known as triple negative breast cancer (TNBC) as it does not express estrogen and progesterone receptors and HER-2. TNBC is an aggressive subtype, and no targeted therapy is available for this subtype. Molecular subtypes of BC differ in their incidence rates, metastasis, aggressiveness and response to a treatment [8]. Molecular profiling of BC subtypes has led to the development of personalized therapies that have notably improved the response to the treatment and an overall survival rate [9].
Breast cancer stem cells
One of the major factors that contributes to difficulty in the overall treatment of BC includes intratumoral heterogeneity (reviewed in [10]). Two theories have been proposed to explain the heterogeneity among cancer subtypes: cancer stem cell (CSC) hypothesis and clonal evolution model. The CSC hypothesis suggests that the CSCs represent a small subpopulation of cells within a tumor that have the ability to divide indefinitely and differentiate into different cell types within the tumor and drive the tumor growth. The CSC hypothesis also claims that the phenotypic hierarchy within the tumor cells is maintained by CSC to promote heterogeneity [11]. Breast CSCs have been defined as tumorigenic cells that can form a tumor efficiently in a NOD/SCID mouse model [12]. Cells within a breast tumor were sorted based on the expressing of cell surface markers CD44 and CD24. Cells that were positive for CD44 and showed a low or no expression of CD24 receptors formed tumors more efficiently as compared to BC cells of other phenotypic characteristics in NOD/SCID mice [12]. However, use of CD44 and CD24 as hallmarks to identify CSCs has been debated, since BC cells with another cell marker, aldehyde dehydrogenase 1 (ALDH1) have also been reported to have tumorigenic potential [13].
The clonal evolution model proposes that a single cancer originator cell accumulates genetic aberrations during the process of carcinogenesis and cancer cells further accumulate random genetic mutations with each cycle of clonal expansion, resulting in the tumor heterogeneity [14, 15]. The clonal evolution model does not support the existence of phenotypic hierarchy within a tumor but rather attributes the intratumoral heterogeneity to natural selection.
Breast cancer therapies
Surgery, chemotherapy and radiotherapy still remain a part of the current standard therapy for BC [2]. Hormone-based therapies use drugs that inhibit estrogen- and/or progesterone-mediated promotion of cancer cell growth. A major hurdle in using hormone-based therapies is that their effectiveness largely depends on the expression of HRs on BC cells. The HR status for estrogen and progesterone is variable among different BC subtypes, and therefore, the degree of dependence on these hormones for cell growth differs significantly [16, 17]. Some of the distinct classes of drugs that fall under hormone-based therapies include selective estrogen receptor (ER) antagonists/modulators and aromatase inhibitors which can control the cancer cell growth mediated through hormones [18].
A range of immunotherapeutics are currently being tested for BC in several studies at both preclinical and clinical levels. Some of the major immune-stimulatory proteins that are being studied for therapeutic application in cancer patients include granulocyte monocyte colony-stimulating factor (GMCSF), granulocyte colony-stimulating factor (GCSF), tumor necrosis factor (TNF), several interleukins (ILs) [IL-2, IL-4, IL-12, IL-24] and interferons (IFNs). Among these cytokines, currently IFN-alpha2a, IFN-alpha2b and IL-2 are approved for treatment of cancer by the US FDA [19]. Additionally, three more cytokines are approved as adjuvant cancer therapeutics, which include TNF, GMCSF and GCSF. The TNF acts as cytotoxic agent for cancer cells, and GMCSF and GCSF act as immune boosters [19].
Targeted therapies are relatively newer treatment option for BC which includes therapeutics directed at specific molecular targets. Targeted therapeutics has drawn attention with encouraging results in both preclinical and clinical studies where they have shown a notable therapeutic efficacy. Some of the key-targeted therapies for BC include anti-HER-2 therapeutics (trastuzumab, pertuzumab), epidermal growth factor receptor (EGFR) inhibitors (erlotinib and gefitinib), mTOR inhibitors (everolimus), cyclin-dependent kinase inhibitors (abemaciclib, ribociclib and palbociclib) and anti-VEGF therapeutics (bevacizumab) [20]. Most of the targeted therapeutics has been shown to inhibit cancer cell growth and metastasis in BC patients when used as an adjuvant therapeutic along with other standard therapies. However, BC subtypes are heterogeneous and a pre-screening of patients for the specific molecular targets may be critical to ensure the effectiveness of targeted therapies. For example, BC subtype such as TNBC represents a challenge for targeted therapies, since TNBC is characterized by the absence of target receptors for estrogen, progesterone as well as HER-2.
OVs are an emerging therapeutic option for BC and have several advantages over other therapeutics. Oncolytic virotherapy (OVT) is based on using viruses that can replicate in cancer cells, causing cell lysis and may also be expressing a range of therapeutic transgenes, including immune-stimulatory factors, apoptotic inducers and prodrug activators.
Oncolytic viruses for breast cancer in preclinical studies
OVs designed for the BC treatment can be broadly divided into two major groups: (a) OVs naturally targeting indigenous defects in BC cells and (b) engineered OVs. Only some viruses have an inherent property to exploit the altered biochemical state in cancer cells, while most other viruses have to be genetically modified to make them oncoselective and/or express therapeutic transgenes.
Oncolytic viruses targeting indigenous defects in breast cancer cells
Newcastle disease virus (NDV) and reovirus target cancer cells with defective IFN and Ras pathways, respectively [21, 22]. Parvovirus-H1 targets cancer cells with dysregulated cell cycle and cellular division [23]. Breast and many other cancer types overexpress CD46 receptors which are used for entry into a cell by the wild-type measles virus (MV) [24–26]. Most cancer cells harbor defects in signaling pathways, resulting in impaired responses to anti-viral cytokines such as IFN I/II and TNF, rendering them more susceptible to viral infection [27]. Irrespective of the native degree of oncoselectivity of a virus, most OVs are capable of exploiting the defective cellular pathways in cancer cells, including the anti-viral protective pathways [27, 28].
Engineered oncolytic viruses for breast cancer treatment
Most of the cancer therapeutics works through either directly targeting and killing cancer cells or causing indirect negative effect on cancer cells by disrupting the TME. OVs have been engineered to kill BC cells directly and/or through disruption of TME [29–31]. Genetic modification of OVs to express a range of transgenes such as immune-stimulatory factors and apoptosis inducers can enhance the cancer cell killing ability of viruses. TME can also be targeted to disrupt the tumor vasculature and inhibit the growth-promoting effect of TME by modifying OVs to express transgenes that are directed at specific targets within the TME. Furthermore, tumor-specific OVs that are engineered to express the prodrug activator transgenes can help limit the toxic effects of chemotherapeutic agents to cancer cells and their vicinity, when these OVs are used in conjunction with chemotherapeutics [32–34].
Oncolytic viruses expressing immune-stimulatory transgenes
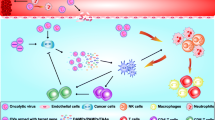
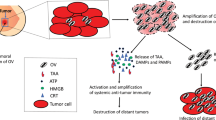
Viruses can induce both innate and adaptive immune responses against cancer cells, even when they are not modified to express any immune-stimulatory transgenes. For example, an attenuated recombinant VV, GL-ONC1/GLV-1h68 armed with no immune-stimulatory transgenes can induce a comprehensive anticancer immune response involving cytokines and chemokines and regress BC tumor xenografts in mice significantly. The GL-ONC1/GLV-1h68 has been modified to increase its tumor selectivity by ablating the viral genes encoding thymidine kinase (TK) and hemagglutinin [35]. However, increasing number of OVs are being genetically modified to express immune-stimulatory transgenes to further boost the anticancer immune response. OVs expressing the immune-stimulatory transgenes exert anti-tumor effects through several other mechanisms, in addition to the direct tumor cell lysis such as: (a) disrupting TME which plays a critical supportive role for cancer cells, (b) targeting uninfected cancer cells within the tumor mass and at distant secondary metastatic sites via induction of an anticancer immune response and (c) providing a long-term anticancer immune response even after discontinuation of the virotherapy (Fig. 1).
Anticancer activity of oncolytic viruses expressing immune-stimulatory transgenes injected intratumorally or intravenously 1 immune cells lead to disruption of tumor microenvironment 2 activated immune cell against cancer antigens can target cancer cells at distant metastatic sites 3 induction of anticancer immune response allows continuous therapeutic benefits even after discontinuation of virotherapy
Although a range of recombinant immune-stimulatory proteins are being investigated as immunotherapeutic agents for different cancer types, the associated systemic toxicity allows use of only low tolerable doses. Engineered OVs carrying immune-stimulatory transgenes allow targeted delivery of immune-stimulatory proteins in cancer cells with reduced systemic toxicity. A range of OVs have been engineered to express ILs to increase their therapeutic efficacy for cancer treatment. An IL-2 expressing NDV [36], IL-4 expressing vesicular stomatitis virus (VSV) [37], IL-12 expressing HSV [38] and IL-24 expressing adenovirus [39] are some of the OVs that have been engineered with a common goal of induction of anticancer immune response through activation and recruitment of T cells, macrophages and natural killer (NK) cells.
Oncolytic viruses inducing apoptotic death in cancer cells
Another engineering strategy for OVs that has been effective in treating BC is through induction of cell death via delivering the pro-apoptotic transgenes in cancer cells. An oncolytic HSV expressing a pro-apoptotic transgene, TNF-related apoptosis-inducing ligand (TRAIL) was able to control TNBC growth effectively in Nu/nu nude mouse model [40]. Upon binding to its receptor, TRAIL activates caspase cascade mediated through pro-caspase 8/10 and induces apoptosis in BC cells [41–43]. Additionally, combination of radiotherapy with TRAIL has been shown to regress the breast tumors more effectively [44]. Radiotherapy can sensitize cancer cells to the TRAIL-mediated apoptosis, resulting in a synergistic therapeutic effect. An oncolytic adenovirus expressing p53 has been shown to induce cell death and restrict tumor growth in breast and other cancer types that are characterized by a null or mutated p53 [45]. Adenovirus expressing p53 has shown an excellent safety profile and can increase the sensitivity of cancer cells to chemotherapy and radiotherapy, even in resistant cancer types by re-stablishing the normal stress response in p53 mutated breast and other cancer cells [46].
Oncolytic viruses targeting tumor vasculature
An improved understanding of the role of TME in promoting tumor growth and metastasis has made the TME an attractive therapeutic target. It has also prompted the development of therapeutic strategies targeting different components of the TME, including the tumor vasculature. Disrupting the tumor vasculature is an effective strategy to control the tumor growth and progression. Oncolytic VV expressing antibody against VEGF has been shown to be effective in controlling the tumor growth through disruption of tumor vasculature, along with viral oncolysis in a mouse model for aggressive BC types such as TNBC [5]. Another oncolytic VV engineered to express C-X-C chemokine receptor type 4 (CXCR4) antagonist can target TME, including the tumor vasculature in breast tumors and has been shown to reduce the tumor metastasis with an improved overall survival [47]. The CXCR4 antagonist prevents the CXCR4/CXCL12 (C-X-C motif chemokine 12) axis-mediated pro-metastatic effect by targeting the TME.
Oncolytic viruses expressing pro-drug activation transgene
Pro-drug-activating OVs allow conversion of pro-drug to active form selectively in cancer cells, causing a maximum damage to cancer cells infected with OVs. Oncolytic adenovirus [32], VV [33] and VSV [34] have been designed to express pro-drug-activating transgenes and assessed for treating BC. Conditionally replicating adenovirus was armed with a suicide gene expressing deoxyribonucleoside kinase of Drosophila melanogaster (Dm-DNK). Adenovirus expressing Dm-DNK has shown an improved efficacy in treating BC when combined with chemotherapeutic pro-drugs (E)-5-(2-bromovinyl)-2′-deoxyuridine (Bvdu) and 2′,2′-difluoro-deoxycytidine (dFdC), both in vitro and in vivo [32]. Oncolytic VV expressing β-galactosidase that can activate the prodrug derived from a seco-analog of the natural antibiotic duocarmycin SA has shown improved therapeutic efficacy in human BC cells [33]. VSV-MΔ51 expressing the cytosine deaminase/uracil phosphoribosyltransferase (CD::UPRT) suicide gene has shown synergistic effect when used with 5-fluorocytosine (5-FC). Combination of VSV-MΔ51 with 5-FC prodrug has been shown to affect the uninfected adjacent tumor cells by releasing toxic metabolites of 5-FC, in vitro [34].
Oncolytic viruses for BC therapy in clinical trials
OVs have shown an impressive therapeutic efficacy when used as a solo treatment agent in several preclinical studies and hence several OVs are currently being tested in clinical trials for safety and efficacy (Table 1). Maneuverability with the genetic engineering techniques when designing OVs has opened up doors for application of multiple treatment strategies. OVs have shown a synergistic effect in combination therapies when used with other treatment options such as chemotherapy, radiotherapy and immunotherapy for treating several cancer types, including BC. Some OVs are also currently under investigation in clinical trials in combination therapies (Table 2).
Oncolytic viruses as monotherapy
A modified tumor-selective oncolytic virus vvDD-CDSR that was generated by ablation of TK and VV growth factor from Western Reserve VV strain was tested in 16 patients with solid tumors, including BC in a phase I clinical trial. Tumor-selective replication and spread of virus to uninfected distant tumor sites with minimal severe adverse effects was reported after the intratumoral injection of vvDD-CDSR (PMID: 25292189) [48]. Oncolytic adenovirus Ad5/3-E2F-Δ24-GMCSF (CGTG-602) can induce a strong anticancer immune response, especially the T cell response against solid tumors in a clinical study involving 13 patients. The Ad5/3-E2F-Δ24-GMCSF is a recombinant adenovirus with an increased tumor specificity that expresses GMCSF. Adenovirus was modified by 24-base pair deletion in the Rb-binding region of the E1A gene and insertion of tumor-specific promoter E2F within the gene which allows expression of E1A gene selectively in cancer cells. Expression of E1A gene in cancer cells results in cellular toxicity and induction of anti-viral immune response (PMID: 25292189) [49]. Reolysin is a serotype 3 strain of a reovirus that targets cancer cells with activated Ras pathway and has shown a good therapeutic efficacy in a clinical trial where it was used as a solo therapeutic intervention in patients with advanced state solid tumors (PMID: 19572105) [50]. Oncolytic adenovirus modified for p16/Rb pathway-based oncoselectivity has been shown to be safe in patients with advanced refractory solid tumors, where further modification of virus to express GMCSF helped in stabilization of disease (PMID: 21630267) [51]. Another oncolytic adenovirus ICOVIR-7 was capable of reducing the tumor burden and stabilizing the disease progression in 5 out of 12 assessable patients with the late-stage refractory solid tumors including breast tumors (PMID: 20501623) [52]. Oncolytic adenovirus telomelysin was used in 16 patients in a clinical study, where 7 evaluable patients showed stabilization of a disease in response to intratumorally injected virus. Adenovirus telomelysin is a modified serotype 5 adenovirus carrying the human telomerase reverse transcriptase promoter (PMID: 19935775) [53]. Oncolytic type 2 adenovirus overexpressing heat shock protein, HSP70, also showed a minimal toxicity with oncolytic effects in both preclinical and clinical studies with a clinical benefit rate of over 48% in a clinical study that recruited 27 patients with advanced solid tumors, including BC (PMID: 19092859) [54]. Oncolytic NDV was better tolerated when patients were desensitized by injecting the initial low dose of virus, followed by injection of high doses of virus, intravenously, in a clinical study involving BC patients, along with other cancer types (PMID: 16638865) [55]. An oncolytic HSV-1, HF10 resulted in marked regression of tumors with no significant adverse effects in patients with metastatic BC, following intratumoral injection of HF10, in a pilot study (PMID: 17346108) [56]. A phase I clinical trial has established the safety profile of oncolytic HSV expressing GMCSF (OncoVEXGM-CSF) that was injected intratumorally in patients with metastatic BC and other cancer types. The OncoVEXGM-CSF induced necrosis of tumor tissue, resulting in reduction of tumor mass (PMID: 17121894) [57].
Oncolytic viruses in combination therapies
Regardless of a phenomenal success of several OVs in preclinical and clinical studies, it is evident that OVs are unlikely to be the sole remedy for treating metastatic disease, especially the advanced-stage cancers. In the advent of several novel cancer therapeutics and their proven benefits and disparate mechanisms of action, one logical way to maximize the efficacy of cancer treatment strategy would be using multimodal cancer therapeutics in combination with achieve synergetic therapeutic benefits. The increasing popularity of the combination therapies is evident by a rise in the number of preclinical and clinical studies testing safety and efficacy of different therapeutic combinations. Combination of low dose cyclophosphamide (CPA) with oncolytic adenovirus can induce an anticancer and anti-viral immune response in BC and other refractory cancers and improved the overall survival [58]. Patients received different dose regimens of CPA intravenously along with the intratumoral injection of oncolytic adenovirus. Use of CPA along with adenovirus did not affect immune response in patients as evident by tumor-specific and virus-specific T cytotoxic (T c) and T helper 1 (T h1) cell response. Additionally, a reduced T regulatory (T reg) cell and T helper 2 (T h2) response was also reported with the use of CPA and oncolytic adenovirus combination therapy. The T reg and T h2 cells are known to play a role in suppressing the anti-tumor immune responses (PMID: 21673660) [58]. A phase I clinical trial using oncolytic adenovirus type c (ONYX-015) along with recombinant human TNF receptor (rhTNFR) dimer showed that the viral clearance from the system can be significantly reduced by impeding human TNF activity. Higher circulating viral loads were observed in patients who received the combination treatment versus who received only virotherapy. The rhTNFR reduces the level of active TNF which avoids a rapid viral clearance from the patient’s system, increasing the therapeutic efficacy of the virus (PMID: 17704755) [59].
Clinical trials investigating OVs for BC treatment will provide the crucial cues for engineering newer OVs. Extensively heterogeneous nature of BC is a major hurdle in determining the optimum treatment strategy which makes it necessary to look for therapeutic targets in cancer cells and TME. Here we discuss some of the major potential therapeutic targets within BC cell and TME that can help designing new OVs aimed at these targets.
Potential therapeutic targets for BC
Chemotherapy is a fundamental part of treatment strategy for BC as it can treat a range of heterogeneous BC subtypes effectively. Some BC cells are indigenously resistant to certain chemotherapeutic agents due to their genetic profiles. Chemoresistant BC cells are characterized by either an absence of specific therapeutic targets or presence of preexisting drug-resistance mechanisms which allow them to survive through the chemotherapy. BC cells that are responsive to certain chemotherapeutic agents can subsequently acquire resistance. In some cases, chemoresistance acquired by BC cells against one drug can provide a cross-protection against additional chemotherapeutics [60]. Although targeted therapeutic agents have shown an inconsistent efficacy in BC patients due to the molecular diversity, it will be important to continue exploring new therapeutic targets to develop alternative treatment options which will be helpful in tackling the resistant BC subtypes.
Some oncogenes such as HER-2 and c-myc have been shown to promote tumor survival and progression [61, 62]. HER-2 or c-myc is overexpressed in several BC cells and has been linked with a decreased overall survival and poor prognosis among patients [61, 63]. In few cases of BC, both HER-2 and c-myc are co-expressed and have been associated with poor prognosis [64]. Moreover, tumor suppressor genes such as BRCA and p53 that are responsible for induction of apoptosis in cells are rendered incompetent in some of the BC subtypes, allowing cancer cells to escape the apoptotic death. Mutations in BRCA have been reported to mediate the anti-apoptotic effect through both p53-mediated and p53-independent mechanisms, which hints at the cross talk between these two tumor suppressor genes [65]. A better understanding of mechanisms involved in the tumor promotion mediated via oncogenes and tumor suppressor genes can help developing new treatment entities.
Cyclins, HRs and poly-[ADP-ribose] polymerases (PARP) are some of the molecular targets of great therapeutic interest in BC. Cyclins are responsible for regulating the cell cycle, and altered cyclins in cancer cells result in an uncontrolled cell replication, which makes cyclins an appealing target [66, 67]. The HRs such as ERs and progesterone receptors have been shown to promote cell growth in cancer cells, and blockade of these receptors can knockout the hormone-mediated cell growth [68, 69]. Defects in the DNA repair mechanism are common in BC cells. PARP plays a crucial role in DNA repair which makes it an attractive therapeutic target [70]. Cellular pathways such as mTOR and insulin-like growth factor-1 receptor (IGF-1R) pathway promote cell survival, growth and metastasis in BC cells [71, 72]. Disruption of these pathways can also help inhibit the growth and progression of the disease in BC patients.
As we understand the increasingly complex and important role played by TME in promoting tumor growth and metastasis, more therapies are being developed that are aimed at therapeutic targets in TME. Stromal tissue in the tumor vicinity is important in the process of neo-angiogenesis which promotes tumor growth and metastasis. The VEGF is critically involved in the process of formation of tumor vasculature and therapeutics antagonizing the action of VEGF can help limit the BC progression [73]. Tumor cells have evolved mechanisms to impede the anti-tumor immune response, some of which are mediated through programmed death–ligand 1 (PD-L1) and cytotoxic T-lymphocyte antigen-4 (CTLA-4). Both PD-L1 and CTLA-4 have been shown to be involved in inhibition of the anti-tumor T cell response [74, 75]. Inhibition of PD-L1 and CTLA-4 induces a strong T cell response against tumor cells that can help in clearing these cells at both primary as well as secondary metastatic sites [76, 77].
Future of oncolytic viruses
Most OVs have demonstrated a good safety profile among cancer patients in several clinical trials. Desensitization to an OV has been used to reduce the treatment toxicity and improve tolerability of OVT in cancer patients by using the two-step process where patients are exposed to a low dose of OV in the first step to minimize the adverse effects of OV during second step [55]. However, exposure to an OV can induce anti-viral immunity in patients which can reduce the therapeutic efficacy of OVs. A serial treatment with OVs that show antigenic discretion can be one of the effective ways to circumvent the loss of therapeutic efficacy of OVs due to a preexisting anti-viral immunity [78].
Safety of OVs in cancer patients following chemotherapy or radiotherapy is one of the prime concerns, since most of these patients are immune-suppressed and it is difficult to predict the toxicity of OVs in these patients. A caution should be exercised when interpreting results of clinical studies. It is very important to consider the status of disease and potential impact of any pre-treatment when evaluating the therapeutic efficacy of OVs in cancer patients. Advanced-stage cancers are known to develop several survival and defense mechanisms that make them relatively difficult to treat [79, 80]. It is easy to over-estimate the efficacy of an OV when the trial involves patients with an early-stage disease, while under-estimate the efficacy when OVs are used to treat patients with advanced-stage cancer. Additionally, the long-term effect of using OVs in terms of toxicity and oncogenic potential need to be assessed, including possibilities of acquisition of mutations by OVs and any possibilities of induction of oncogenesis by OVs.
Genetic modifications of OVs and use of carrier cells, infected ex vivo, can improve the OV delivery in cancer cells and increase the efficacy of OVT [27]. However, we still have a long way to go when it comes to increasing the oncoselectivity of OV candidates. Many OVs have shown a synergistic effect when used along with other therapeutics in both preclinical and clinical studies. However, it will be important to ensure that the therapeutic agent used in combination with OVs does not affect the viral replication, delivery of virus in cancer cells, and induction of anticancer and/or anti-viral immune responses. Assessment of toxicity of combination therapy is critical, since toxic effects associated with each of the therapeutic agents in the combination regimen accrue and can lead to severe cumulative toxicity even at lower dosages.
Viruses have evolved multiple strategies to modulate and neutralize anti-viral immune responses by host following infection. Viruses encode a range of proteins that mimic immune-stimulatory ligands or their receptors that impede the anti-viral immune response, allowing a successful viral replication and disease manifestation [81, 82]. Many OVs have been engineered by ablation of viral genes that are responsible for their tumor tropism and/or reduction in virulence. It would be important to continue the exploration of virus encoded immune-modulatory genes and the effects of these gene ablations on therapeutic efficacy and safety of OVs. Additionally, some viral genes encode tumor-promoting growth factors which can be deleted to abolish the pro-tumor effects of these growth factors. For example, a tanapoxvirus recombinant with a neuregulin mimicking gene deletion was able to regress melanoma xenografts in a nude mouse model [83]. Deletion of a neuregulin mimicking gene from tanapoxvirus abolished its growth-promoting effect on melanoma cells, causing significant tumor regression.
Intratumoral injection of OVs is an efficient way of targeted delivery of OVs; however, this approach will be less feasible and ineffective when treating patients with advanced metastatic disease or with multiple metastatic tumors as the OV delivery to distant metastatic sites can be a big hurdle. Additionally, intratumoral injections are a cumbersome job when tumors are deep seated. A more practical approach when developing new OVs would be to design OVs that can be delivered systemically, allowing a better control over metastatic disease and administration of OVT in an out-patient setting. Most OVs are replication competent mutated viruses, and patients treated will release virus particles in their vicinity. The impact of virus released by cancer patients in their vicinity after OVT on public health, especially immunocompromised, pediatric and geriatric population and environment should be considered seriously and attempts should be made to minimize their effect [84].
Conclusion
In conclusion, the reviewed preclinical studies and clinical trial data testing OVs in BC show that OVs are likely to be an integral part of BC therapy. OVs are especially important when treating BC subtypes like TNBC that respond poorly to chemotherapy in many cases. Other cancer therapeutics has been shown to work with OVs in combination therapies to achieve a synergistic therapeutic effect with a least effect on viral replication and/or induction of immune response [58]. Cues should be taken from the outcomes of clinical trials testing OVs in combination therapies to determine the future cancer therapeutic strategies, including engineering of new OVs. Moreover, a better understanding of the exact roles and mechanisms through which the potential therapeutic targets within cancer cells and TME promote tumor progression will be helpful in designing new OVs aiming these targets. As the personalized cancer therapy becomes a reality, identification of therapeutic targets in cancer cells will help to match the suitable OVs with patients. Patients have been shown to tolerate oncolytic NDV when they are desensitized to the virus by injecting low doses of virus before being injected large doses of the virus [55]. However, the specific anti-viral immune response developed following the first exposure to the OV may lead to a rapid immune clearance of the OV during the subsequent OVT cycles, reducing their efficacy. Serial treatment using antigenically distinct OVs has been shown to enhance the therapeutic efficacy of OVT [78]. In the absence of the effective techniques to maintain the immune tolerance against OVs in cancer patients, a large bank of anti-BC viruses will be required for an effective OVT.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2016;66(1):7–30.
Hunter P. The fourth front against cancer. EMBO Rep. 2011;12(8):769–71.
Pol J, Kroemer G, Galluzzi L. First oncolytic virus approved for melanoma immunotherapy. Oncoimmunology. 2016;5(1):e1115641.
Garber K. China approves world’s first oncolytic. J Natl Cancer Inst. 2006;98(5):298–300.
Gholami S, Marano A, Chen NG, Aguilar RJ, Frentzen A, Chen CH, et al. A novel vaccinia virus with dual oncolytic and anti-angiogenic therapeutic effects against triple-negative breast cancer. Breast Cancer Res Treat. 2014;148(3):489–99.
Weigelt B, Geyer FC, Reis-Filho JS. Histological types of breast cancer: How special are they? Mol Oncol. 2010;4(3):192–208.
Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19(5):403–10.
Polyak K. Heterogeneity in breast cancer. J Clin Investig. 2011;121(10):3786–8.
Perez EA. Breast cancer management: opportunities and barriers to an individualized approach. Oncologist. 2011;16(Supplement 1):20–2.
Martelotto LG, Ng CK, Piscuoglio S, Weigelt B, Reis-Filho JS. Breast cancer intra-tumor heterogeneity. Breast Cancer Res. 2014;16:R48.
Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501(7467):328–37.
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci. 2003;100(7):3983–8.
Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1(5):555–67.
Marusyk A, Polyak K. Tumor heterogeneity: causes and consequences. Biochim Biophys Acta Rev Cancer. 2010;1805(1):105–17.
Merlo LMF, Pepper JW, Reid BJ, Maley CC. Cancer as an evolutionary and ecological process. Nat Rev Cancer. 2006;6(12):924–35.
Davis BW, Zava DT, Locher GW, Goldhirsch A, Hartmann WH. Receptor heterogeneity of human breast cancer as measured by multiple intratumoral assays of estrogen and progesterone receptor. Eur J Cancer Clin Oncol. 1984;20(3):375–82.
Babayan A, Hannemann J, Spotter J, Muller V, Pantel K, Joosse SA. Heterogeneity of estrogen receptor expression in circulating tumor cells from metastatic breast cancer patients. PLoS ONE. 2013;8(9):e75038.
Lumachi F, Luisetto G, Basso SM, Basso U, Brunello A, Camozzi V. Endocrine therapy of breast cancer. Curr Med Chem. 2011;18(4):513–22.
Vacchelli E, Aranda F, Bloy N, Buqué A, Cremer I, Eggermont A, et al. Trial watch—immunostimulation with cytokines in cancer therapy. Oncoimmunology. 2016;5(2):e1115942.
Venur V, Leone J. Targeted therapies for brain metastases from breast cancer. Int J Mol Sci. 2016;17(9):1543.
Errington F, White CL, Twigger KR, Rose A, Scott K, Steele L, et al. Inflammatory tumour cell killing by oncolytic reovirus for the treatment of melanoma. Gene Ther. 2008;15(18):1257–70.
Phuangsab A, Lorence RM, Reichard KW, Peeples ME, Walter RJ. Newcastle disease virus therapy of human tumor xenografts: antitumor effects of local or systemic administration. Cancer Lett. 2001;172(1):27–36.
Dupressoir T, Vanacker JM, Cornells JJ, Duponchel N, Rommelaere J. Inhibition by parvovirus H-1 of the formation of tumors in nude mice and colonies in vitro by transformed human mammary epithelial cells. Cancer Res. 1989;49(12):3203–8.
Anderson BD, Nakamura T, Russell SJ, Peng KW. High CD46 receptor density determines preferential killing of tumor cells by oncolytic measles virus. Cancer Res. 2004;64(14):4919–26.
Iankov ID, Msaouel P, Allen C, Federspiel MJ, Bulur PA, Dietz AB, et al. Demonstration of anti-tumor activity of oncolytic measles virus strains in a malignant pleural effusion breast cancer model. Breast Cancer Res Treat. 2010;122(3):745–54.
McDonald CJ, Erlichman C, Ingle JN, Rosales GA, Allen C, Greiner SM, et al. A measles virus vaccine strain derivative as a novel oncolytic agent against breast cancer. Breast Cancer Res Treat. 2006;99(2):177–84.
Bell J, McFadden G. Viruses for tumor therapy. Cell Host Microbe. 2014;15(3):260–5.
Kim M. Replicating poxviruses for human cancer therapy. J Microbiol. 2015;53(4):209–18.
Cody JJ, Hurst DR. Promising oncolytic agents for metastatic breast cancer treatment. Oncolytic Virother. 2015;4:63–73.
Bramante S, Koski A, Liikanen I, Vassilev L, Oksanen M, Siurala M, et al. Oncolytic virotherapy for treatment of breast cancer, including triple-negative breast cancer. Oncoimmunology. 2016;5(2):e1078057.
Hartkopf AD, Fehm T, Wallwiener D, Lauer UM. Oncolytic virotherapy of breast cancer. Gynecol Oncol. 2011;123(1):164–71.
Dong X, Qu W, Ma S, Zhu Z, Zheng C, He A, et al. Potent antitumoral effects of targeted promoter-driven oncolytic adenovirus armed with Dm-dNK for breast cancer in vitro and in vivo. Cancer Lett. 2013;328(1):95–103.
Seubert CM, Stritzker J, Hess M, Donat U, Sturm JB, Chen N, et al. Enhanced tumor therapy using vaccinia virus strain GLV-1h68 in combination with a beta-galactosidase-activatable prodrug seco-analog of duocarmycin SA. Cancer Gene Ther. 2011;18(1):42–52.
Leveille S, Samuel S, Goulet M-L, Hiscott J. Enhancing VSV oncolytic activity with an improved cytosine deaminase suicide gene strategy. Cancer Gene Ther. 2011;18(6):435–43.
Zhang Q, Yu YA, Wang E, Chen N, Danner RL, Munson PJ, et al. Eradication of solid human breast tumors in nude mice with an intravenously injected light-emitting oncolytic vaccinia virus. Cancer Res. 2007;67(20):10038–46.
Wu Y, He J, An Y, Wang X, Liu Y, Yan S, et al. Recombinant Newcastle disease virus (NDV/Anh-IL-2) expressing human IL-2 as a potential candidate for suppresses growth of hepatoma therapy. J Pharmacol Sci. 2015;24–30.
Fernandez M, Porosnicu M, Markovic D, Barber GN. Genetically engineered vesicular stomatitis virus in gene therapy: application for treatment of malignant disease. J Virol. 2002;76(2):895–904.
Liu R, Varghese S, Rabkin SD. Oncolytic herpes simplex virus vector therapy of breast cancer in C3(1)/SV40 T-antigen transgenic mice. Cancer Res. 2005;65(4):1532–40.
Sarkar D, Su Z-Z, Vozhilla N, Park ES, Gupta P, Fisher PB. Dual cancer-specific targeting strategy cures primary and distant breast carcinomas in nude mice. Proc Natl Acad Sci USA. 2005;102(39):14034–9.
Zhu W, Zhang H, Shi Y, Song M, Zhu B, Wei L. Oncolytic adenovirus encoding tumor necrosis factor-related apoptosis inducing ligand (TRAIL) inhibits the growth and metastasis of triple-negative breast cancer. Cancer Biol Ther. 2014;14(11):1016–23.
Gazitt Y. TRAIL is a potent inducer of apoptosis in myeloma cells derived from multiple myeloma patients and is not cytotoxic to hematopoietic stem cells. Leukemia. 1999;13(11):1817–24.
Pollack IF, Erff M, Ashkenazi A. Direct stimulation of apoptotic signaling by soluble Apo2l/tumor necrosis factor-related apoptosis-inducing ligand leads to selective killing of glioma cells. Clin Cancer Res. 2001;7(5):1362–9.
Ashkenazi A. Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat Rev Cancer. 2002;2(6):420–30.
Chinnaiyan AM, Prasad U, Shankar S, Hamstra DA, Shanaiah M, Chenevert TL, et al. Combined effect of tumor necrosis factor-related apoptosis-inducing ligand and ionizing radiation in breast cancer therapy. Proc Natl Acad Sci USA. 2000;97(4):1754–9.
Nielsen LL, Dell J, Maxwell E, Armstrong L, Maneval D, Catino JJ. Efficacy of p53 adenovirus-mediated gene therapy against human breast cancer xenografts. Cancer Gene Ther. 1996;4(2):129–38.
Roth JA. Adenovirus p53 gene therapy. Expert Opin Biol Ther. 2006;6(1):55–61.
Gil M, Seshadri M, Komorowski MP, Abrams SI, Kozbor D. Targeting CXCL12/CXCR4 signaling with oncolytic virotherapy disrupts tumor vasculature and inhibits breast cancer metastases. Proc Natl Acad Sci USA. 2013;110(14):E1291–300.
Zeh HJ, Downs-Canner S, McCart JA, Guo ZS, Rao UNM, Ramalingam L, et al. First-in-man study of western reserve strain oncolytic vaccinia virus: safety, systemic spread, and antitumor activity. Mol Ther. 2015;23(1):202–14.
Hemminki O, Parviainen S, Juhila J, Turkki R, Linder N, Lundin J, et al. Immunological data from cancer patients treated with Ad5/3-E2F-DELTA24-GMCSF suggests utility for tumor immunotherapy. Oncotarget. 2015;6(6):4467–81.
Gollamudi R, Ghalib MH, Desai KK, Chaudhary I, Wong B, Einstein M, et al. Intravenous administration of Reolysin, a live replication competent RNA virus is safe in patients with advanced solid tumors. Investig N Drugs. 2010;28(5):641–9.
Pesonen S, Diaconu I, Cerullo V, Escutenaire S, Raki M, Kangasniemi L, et al. Integrin targeted oncolytic adenoviruses Ad5-D24-RGD and Ad5-RGD-D24-GMCSF for treatment of patients with advanced chemotherapy refractory solid tumors. Int J Cancer. 2012;130(8):1937–47.
Nokisalmi P, Pesonen S, Escutenaire S, Särkioja M, Raki M, Cerullo V, et al. Oncolytic adenovirus ICOVIR-7 in patients with advanced and refractory solid tumors. Clin Cancer Res. 2010;16(11):3035–43.
Nemunaitis J, Tong AW, Nemunaitis M, Senzer N, Phadke AP, Bedell C, et al. A phase I study of telomerase-specific replication competent oncolytic adenovirus (telomelysin) for various solid tumors. Mol Ther. 2010;18(2):429–34.
Li J-L, Liu H-L, Zhang X-R, Xu J-P, Hu W-K, Liang M, et al. A phase I trial of intratumoral administration of recombinant oncolytic adenovirus overexpressing HSP70 in advanced solid tumor patients. Gene Ther. 2009;16(3):376–82.
Laurie SA, Bell JC, Atkins HL, Roach J, Bamat MK, O’Neil JD, et al. A phase 1 clinical study of intravenous administration of PV701, an oncolytic virus, using two-step desensitization. Clin Cancer Res. 2006;12(8):2555–62.
Nakao A, Takeda S, Shimoyama S, Kasuya H, Kimata H, Teshigahara O, et al. Clinical experiment of mutant herpes simplex virus HF10 therapy for cancer. Curr Cancer Drug Targets. 2007;7(2):169–74.
Hu JCC, Coffin RS, Davis CJ, Graham NJ, Groves N, Guest PJ, et al. A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin Cancer Res. 2006;12(22):6737–47.
Cerullo V, Diaconu I, Kangasniemi L, Rajecki M, Escutenaire S, Koski A, et al. Immunological effects of low-dose cyclophosphamide in cancer patients treated with oncolytic adenovirus. Mol Ther. 2011;19(9):1737–46.
Nemunaitis J, Senzer N, Sarmiento S, Zhang Y-A, Arzaga R, Sands B, et al. A phase I trial of intravenous infusion of ONYX-015 and enbrel in solid tumor patients. Cancer Gene Ther. 2007;14(11):885–93.
Longley DB, Johnston PG. Molecular mechanisms of drug resistance. J Pathol. 2005;205(2):275–92.
Das S, Sondarva G, Viswakarma N, Nair RS, Osipo C, Tzivion G, et al. Human epidermal growth factor receptor 2 (HER2) impedes MLK3 kinase activity to support breast cancer cell survival. J Biol Chem. 2015;290(35):21705–12.
Xu J, Chen Y, Olopade OI. MYC and breast cancer. Genes Cancer. 2010;1(6):629–40.
Latta EK, Tjan S, Parkes RK, O’Malley FP. The role of HER2/neu overexpression/amplification in the progression of ductal carcinoma in situ to invasive carcinoma of the breast. Mod Pathol. 2002;15(12):1318–25.
Aulmann S, Bentz M, Sinn HP. C-myc oncogene amplification in ductal carcinoma in situ of the breast. Breast Cancer Res Treat. 2002;74(1):25–31.
Zhang H, Somasundaram K, Peng Y, Tian H, Zhang H, Bi D, et al. BRCA1 physically associates with p53 and stimulates its transcriptional activity. Oncogene. 1998;16(13):1713–21.
Casimiro MC, Crosariol M, Loro E, Li Z, Pestell RG. Cyclins and cell cycle control in cancer and disease. Genes Cancer. 2012;3(11–12):649–57.
Keyomarsi K, O’Leary N, Molnar G, Lees E, Fingert HJ, Pardee AB. Cyclin E, a potential prognostic marker for breast cancer. Cancer Res. 1994;54(2):380–5.
Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med. 2011;62:233–47.
Osborne CK, Schiff R. Estrogen-receptor biology: continuing progress and therapeutic implications. J Clin Oncol. 2005; 23(8):1616–22.
De Vos M, Schreiber V, Dantzer F. The diverse roles and clinical relevance of PARPs in DNA damage repair: current state of the art. Biochem Pharmacol. 2012;84(2):137–46.
Farabaugh SM, Boone DN, Lee AV. Role of IGF1R in breast cancer subtypes, stemness, and lineage differentiation. Front Endocrinol (Lausanne). 2015;6(April):59.
Khan M, Biswas D, Ghosh M, Mandloi S, Chakrabarti S, Chakrabarti P. mTORC2 controls cancer cell survival by modulating gluconeogenesis. Cell Death Discov. 2015;1(July):15016.
Klement G, Huang P, Mayer B, Green SK, Man S, Bohlen P, et al. Differences in therapeutic indexes of combination metronomic chemotherapy and an anti-VEGFR-2 antibody in multidrug-resistant human breast cancer xenografts. Clin Cancer Res. 2002;8(1):221–32.
Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206(13):3015–29.
Contardi E, Palmisano GL, Tazzari PL, Martelli AM, Falà F, Fabbi M, et al. CTLA-4 is constitutively expressed on tumor cells and can trigger apoptosis upon ligand interaction. Int J Cancer. 2005;117(4):538–50.
Chen H, Liakou CI, Kamat A, Pettaway C, Ward JF, Tang DN, et al. Anti-CTLA-4 therapy results in higher CD4 + ICOShi T cell frequency and IFN-gamma levels in both nonmalignant and malignant prostate tissues. Proc Natl Acad Sci USA. 2009;106(8):2729–34.
Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007;19(7):813–24.
Zhang Y-Q, Tsai Y-C, Monie A, Wu T-C, Hung C-F. Enhancing the therapeutic effect against ovarian cancer through a combination of viral oncolysis and antigen-specific immunotherapy. Mol Ther. 2010;18(4):692–9.
Gajewski TF, Schreiber H, Fu Y-X. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14(10):1014–22.
Igney FH, Krammer PH. Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer. 2002;2(4):277–88.
Finlay BB, McFadden G. Anti-immunology: evasion of the host immune system by bacterial and viral pathogens. Cell. 2006;124(4):767–82.
Seet BT, Johnston JBB, Brunetti CR, Barrett JW, Everett H, Cameron C, et al. Poxviruses and immune evasion. Annu Rev Immunol. 2003;21(1):377–423.
Zhang T, Suryawanshi YR, Kordish DH, Woyczesczyk HM, Jeng D, Essani K. Tanapoxvirus lacking a neuregulin-like gene regresses human melanoma tumors in nude mice. Virus Genes. 2016. doi:10.1007/s11262-016-1402-2.
Buijs PR, Verhagen JH, van Eijck CH, van den Hoogen BG. Oncolytic viruses: from bench to bedside with a focus on safety. Hum Vaccines Immunother. 2015;11(7):1573–84.
Acknowledgements
We are grateful to Rob Eversole, David Jeng, Sandhya Hasure and Farzad Razi for editorial comments. Funding was provided by Western Michigan University Fund 23.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Suryawanshi, Y.R., Zhang, T. & Essani, K. Oncolytic viruses: emerging options for the treatment of breast cancer. Med Oncol 34, 43 (2017). https://doi.org/10.1007/s12032-017-0899-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-017-0899-0