Abstract
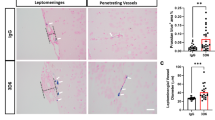
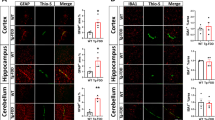
Cerebrovascular amyloidosis caused by amyloid accumulation in blood vessel walls may lead to hemorrhagic stroke and cognitive impairment. Expression of TGF-β1 under glial fibrillary acidic protein promoter in mice leads to age-related deposition of amyloid, including β-amyloid (Aβ), around cerebral blood vessels, leading to vascular pathology starting at age of 7 months. We have recently shown the important role of macrophages in clearing cerebrovascular amyloid. Scavenger receptor A (SRA) is a multi-ligand and multifunctional receptor expressed on macrophages, and it has been suggested to play a role in meditating phagocytosis of different types of antigens. We investigated the role of SRA in mediating cerebrovascular amyloid clearance. We bred TGF-β1 mice with SRA−/− mice and discovered that TGF-β1/SRA−/− mice showed cerebrovascular pathology at an earlier age (3 months) compared with TGF-β1 mice. Furthermore, SRA deficiency in macrophages led to impaired clearing of congophilic cerebrovascular amyloid from amyloid precursor protein mouse model and led to reduced phagocytosis of both soluble and insoluble Aβ in vivo as compared with macrophages from wild-type mice. Our findings demonstrate the important role of SRA in cerebrovascular amyloid pathology and suggest targeting SRA for future diagnostic and therapeutic approaches for cerebral amyloid angiopathy.



Similar content being viewed by others
References
Christie RH, Freeman M et al (1996) Expression of the macrophage scavenger receptor, a multifunctional lipoprotein receptor, in microglia associated with senile plaques in Alzheimer’s disease. Am J Pathol 148(2):399–403
Chung H, Brazil MI et al (2001) Uptake of fibrillar beta-amyloid by microglia isolated from MSR-A (type I and type II) knockout mice. NeuroReport 12(6):1151–1154
El Khoury J, Hickman SE et al (1996) Scavenger receptor-mediated adhesion of microglia to beta-amyloid fibrils. Nature 382(6593):716–719
Frenkel D, Maron R et al (2005) Nasal vaccination with a proteosome-based adjuvant and glatiramer acetate clears beta-amyloid in a mouse model of Alzheimer disease. J Clin Invest 115(9):2423–2433
Frenkel D, Puckett L et al (2008) A nasal proteosome adjuvant activates microglia and prevents amyloid deposition. Ann Neurol 63(5):591–601
Greenberg SM, Gurol ME et al (2004) Amyloid angiopathy-related vascular cognitive impairment. Stroke 35(11 Suppl 1):2616–2619
Hawkes CA, McLaurin J (2009) Selective targeting of perivascular macrophages for clearance of beta-amyloid in cerebral amyloid angiopathy. Proc Natl Acad Sci U S A 106(4):1261–1266
Hickman SE, Allison EK et al (2008) Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer’s disease mice. J Neurosci 28(33):8354–8360
Krieger M (1997) The other side of scavenger receptors: pattern recognition for host defense. Curr Opin Lipidol 8(5):275–280
Levy-Barazany H, Frenkel D (2012) Expression of scavenger receptor A on antigen presenting cells is important for CD4+ T-cells proliferation in EAE mouse model. J Neuroinflammation 9:120
Lifshitz V, Weiss R et al (2012) Immunotherapy of cerebrovascular amyloidosis in a transgenic mouse model. Neurobiol Aging 33(2):432 e1–432 e13
Nicoll JA, Yamada M et al (2004) Cerebral amyloid angiopathy plays a direct role in the pathogenesis of Alzheimer’s disease. Pro-CAA position statement. Neurobiol Aging 25(5):589–597, discussion 603-584
Oakley H, Cole SL et al (2006) Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. J Neurosci 26(40):10129–10140
Paresce DM, Ghosh RN et al (1996) Microglial cells internalize aggregates of the Alzheimer’s disease amyloid beta-protein via a scavenger receptor. Neuron 17(3):553–565
Platt N, Gordon S (2001) Is the class A macrophage scavenger receptor (SR-A) multifunctional?—The mouse’s tale. J Clin Invest 108(5):649–654
Ruan L, Kang Z et al (2009) Amyloid deposition and inflammation in APPswe/PS1dE9 mouse model of Alzheimer’s disease. Curr Alzheimer Res 6(6):531–540
Thanopoulou K, Fragkouli A et al (2010) Scavenger receptor class B type I (SR-BI) regulates perivascular macrophages and modifies amyloid pathology in an Alzheimer mouse model. Proc Natl Acad Sci U S A 107(48):20816–20821
Weiss R, Lifshitz V et al (2011) TGF-beta1 affects endothelial cell interaction with macrophages and T cells leading to the development of cerebrovascular amyloidosis. Brain Behav Immun 25(5):1017–1024
Winkler DT, Bondolfi L et al (2001) Spontaneous hemorrhagic stroke in a mouse model of cerebral amyloid angiopathy. J Neurosci 21(5):1619–1627
Wyss-Coray T, Feng L et al (1995) Increased central nervous system production of extracellular matrix components and development of hydrocephalus in transgenic mice overexpressing transforming growth factor-beta 1. Am J Pathol 147(1):53–67
Wyss-Coray T, Lin C et al (2000a) Chronic overproduction of transforming growth factor-beta1 by astrocytes promotes Alzheimer’s disease-like microvascular degeneration in transgenic mice. Am J Pathol 156(1):139–150
Wyss-Coray T, Lin C et al (2000b) Alzheimer’s disease-like cerebrovascular pathology in transforming growth factor-beta 1 transgenic mice and functional metabolic correlates. Ann N Y Acad Sci 903:317–323
Zlokovic BV (2005) Neurovascular mechanisms of Alzheimer’s neurodegeneration. Trends Neurosci 28(4):202–208
Acknowledgments
We wish to thank Pro. Tony Wyss-Coray from Stanford University School of Medicine, USA, for providing TGF-β1 mice. This work is supported by grants from the Alzheimer's Association NIRG-11-205535 and ISF (to D.F.).
Conflict of Interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lifshitz, V., Weiss, R., Levy, H. et al. Scavenger Receptor A Deficiency Accelerates Cerebrovascular Amyloidosis in an Animal Model. J Mol Neurosci 50, 198–203 (2013). https://doi.org/10.1007/s12031-012-9909-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-012-9909-z