Abstract
Purpose
Epithelial-mesenchymal transition (EMT) is a biological dynamic process by which epithelial cells lose their epithelial phenotype and acquire mesenchymal invasive and migratory characteristics. This has been postulated as an essential step during cancer progression and metastasis. Although this is well described in other tumors, the role of EMT in adrenocortical tumors (ACT) has yet to be addressed.
Methods
The aim of this study was to evaluate the expression of EMT markers e-cadherin, vimentin, and fibronectin, along with EMT-transcription factors (EMT-TFs), TWIST1, SIP1, and SNAIL in 24 adrenocortical carcinoma (ACC), 19 adrenocortical adenomas (ACA), 27 childhood-onset adrenocortical tumors (CAT), and 12 normal adrenal glands. The association of EMT and EMT-TFs with clinical outcomes and pathology features were also evaluated.
Results
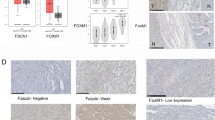
Cytoplasmic vimentin expression was increased among CAT samples when compared to ACC, ACA, and normal adrenal samples (p < 0.001). There was no difference in e-cadherin and fibronectin expression observed between groups. Nuclear and cytoplasmic expression of TWIST1 and SIP1 was stronger in CAT and ACC vs. ACA and normal tissue samples (all, p < 0.05). ACT, regardless of classification, exhibited increased SNAIL expression when compared to normal tissue (p < 0.05). A significant correlation was observed between vimentin and TWIST1 (r s = 0.44, p < 0.001); SIP1 (r s = 0.51, p < 0.001); and SNAIL (r s = 0.23, p < 0.05). TWIST1 and SIP1 expressions demonstrated a significant correlation (r s = 0.56, p < 0.001). High SIP1 expression was associated with a lower survival rate among ACC cases (p < 0.05).
Conclusions
Vimentin, TWIST1, and SIP1 expressions are increased in aggressive ACT. Therefore, EMT may play a relevant role in adrenal tumorigenesis.





Similar content being viewed by others
References
G. Mansmann, J. Lau, E. Balk, M. Rothberg, Y. Miyachi, S.R. Bornstein, The clinically inapparent adrenal mass: update in diagnosis and management. Endocr. Rev. 25(2), 309–340 (2004)
B. Allolio, M. Fassnacht, Clinical review: Adrenocortical carcinoma: clinical update. J. Clin. Endocrinol. Metab. 91(6), 2027–2037 (2006)
B.C. Figueiredo, R. Sandrini, G.P. Zambetti, R.M. Pereira, C. Cheng, W. Liu, L. Lacerda, M.A. Pianovski, E. Michalkiewicz, J. Jenkins, C. Rodriguez-Galindo, M.J. Mastellaro, S. Vianna, F. Watanabe, F. Sandrini, S.B. Arram, P. Boffetta, R.C. Ribeiro, Penetrance of adrenocortical tumours associated with the germline TP53 R337H mutation. J. Med. Genet. 43(1), 91–96 (2006)
J.D. Wasserman, A. Novokmet, C. Eichler-Jonsson, R.C. Ribeiro, C. Rodriguez-Galindo, G.P. Zambetti, D. Malkin, Prevalence and functional consequence of TP53 mutations in pediatric adrenocortical carcinoma: a children’s oncology group study. J. Clin. Oncol. 33(6), 602–609 (2015)
D. Bulzico, P.A. de Faria, M.P. de Paula, M.A. Bordallo, C.H. Pessoa, R. Corbo, S. Ferman, M. Vaisman, L.V. Neto, Recurrence and mortality prognostic factors in childhood adrenocortical tumors: analysis from the Brazilian National Institute of Cancer experience. Pediatr. Hematol. Oncol. 33(4), 248–258 (2016)
D. Bulzico, P.A. Faria, M.P. De Paula, F. Vaisman, C.H. Pessoa, B. Vilhena, R. Corbo, M. Vaisman, L. Vieira Neto, Recurrence and mortality in adulthood adrenocortical tumors: analysis from the Brazilian National Institute of Cancer experience. Cancer Res. Front. 2(3), 368–379 (2016). https://doi.org/10.17980/2016.368
M. Fassnacht, M. Kroiss, B. Allolio, Update in adrenocortical carcinoma. J. Clin. Endocrinol. Metab. 98(12), 4551–4564 (2013)
E.D. Hay, An overview of epithelio-mesenchymal transformation. Acta. Anat. 154(1), 8–20 (1995)
M. Zeisberg, E.G. Neilson, Biomarkers for epithelial-mesenchymal transitions. J. Clin. Invest. 119(6), 1429–1437 (2009)
R. Kalluri, E.G. Neilson, Epithelial-mesenchymal transition and its implications for fibrosis. J. Clin. Invest. 112(12), 1776–1784 (2003)
R. Kalluri, R.A. Weinberg, The basics of epithelial-mesenchymal transition. J. Clin. Invest. 119(6), 1420–1428 (2009)
V. Bolos, H. Peinado, M.A. Perez-Moreno, M.F. Fraga, M. Esteller, A. Cano, The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J. Cell. Sci. 116(Pt 3), 499–511 (2003)
A. Cano, M.A. Perez-Moreno, I. Rodrigo, A. Locascio, M.J. Blanco, M.G. del Barrio, F. Portillo, M.A. Nieto, The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat. Cell. Biol. 2(2), 76–83 (2000)
C. Vandewalle, J. Comijn, B. De Craene, P. Vermassen, E. Bruyneel, H. Andersen, E. Tulchinsky, F. Van Roy, G. Berx, SIP1/ZEB2 induces EMT by repressing genes of different epithelial cell-cell junctions. Nucleic Acids Res. 33(20), 6566–6578 (2005)
J. Yang, S.A. Mani, J.L. Donaher, S. Ramaswamy, R.A. Itzykson, C. Come, P. Savagner, I. Gitelman, A. Richardson, R.A. Weinberg, Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 117(7), 927–939 (2004)
Y.H. Kong, S.N. Syed Zanaruddin, S.H. Lau, A. Ramanathan, T.G. Kallarakkal, V.K. Vincent-Chong, W.M. Wan Mustafa, M.T. Abraham, Z.A. Abdul Rahman, R.B. Zain, S.C. Cheong, Co-expression of TWIST1 and ZEB2 in oral squamous cell carcinoma is associated with poor survival. PLoS ONE 10(7), e0134045 (2015)
J.M. Lee, S. Dedhar, R. Kalluri, E.W. Thompson, The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J. Cell. Biol. 172(7), 973–981 (2006)
D. Medici, E.D. Hay, B.R. Olsen, Snail and slug promote epithelial-mesenchymal transition through beta-catenin-T-cell factor-4-dependent expression of transforming growth factor-beta3. Mol. Biol. Cell. 19(11), 4875–4887 (2008)
K. Nordfors, J. Haapasalo, K. Makela, K.J. Granberg, M. Nykter, M. Korja, T. Paavonen, H. Haapasalo, Y. Soini, Twist predicts poor outcome of patients with astrocytic glioma. J. Clin. Pathol. 68(11), 905–912 (2015)
Y. Soini, H. Tuhkanen, R. Sironen, I. Virtanen, V. Kataja, P. Auvinen, A. Mannermaa, V.M. Kosma, Transcription factors zeb1, twist and snai1 in breast carcinoma. BMC Cancer 11, 73 (2011)
H. Sui, L. Zhu, W. Deng, Q. Li, Epithelial-mesenchymal transition and drug resistance: role, molecular mechanisms, and therapeutic strategies. Oncol. Res. Treat 37(10), 584–589 (2014)
Y. Xu, B. Hu, L. Qin, L. Zhao, Q. Wang, J. Jiang, SRC-1 and Twist1 expression positively correlates with a poor prognosis in human breast cancer. Int. J. Biol. Sci. 10(4), 396–403 (2014)
M.H. Yang, C.L. Chen, G.Y. Chau, S.H. Chiou, C.W. Su, T.Y. Chou, W.L. Peng, J.C. Wu, Comprehensive analysis of the independent effect of twist and snail in promoting metastasis of hepatocellular carcinoma. Hepatology 50(5), 1464–1474 (2009)
P. Zhang, P. Hu, H. Shen, J. Yu, Q. Liu, J. Du, Prognostic role of Twist or Snail in various carcinomas: A systematic review and meta-analysis. Eur. J. Clin. Invest. 44(11), 1072–1094 (2014)
Q.Q. Zhu, C. Ma, Q. Wang, Y. Song, T. Lv, The role of TWIST1 in epithelial-mesenchymal transition and cancers. Tumour Biol. 37(1), 185–197 (2016)
L.M. Weiss, L.J. Medeiros, A.L. Vickery Jr., Pathologic features of prognostic significance in adrenocortical carcinoma. Am. J. Surg. Pathol. 13(3), 202–206 (1989)
S.A. Fuhrman, L.C. Lasky, C. Limas, Prognostic significance of morphologic parameters in renal cell carcinoma. Am. J. Surg. Pathol. 6(7), 655–663 (1982)
R. Sandrini, R.C. Ribeiro, L. DeLacerda, Childhood adrenocortical tumors. J. Clin. Endocrinol. Metab. 82(7), 2027–2031 (1997)
D. Bulzico, D.C. Torres, G.M. Ferreira, B.R.B. Pires, P.A.S. de Faria, R. Hassan, E. Abdelhay, M. Vaisman, L. Vieira Neto, A Novel TP53 mutation associated with TWIST1 and SIP1 expression in an aggressive adrenocortical carcinoma. Endocr. Pathol. (2017). https://doi.org/10.1007/s12022-017-9482-7
L. Zhou, L. Yu, S. Wu, Z. Feng, W. Song, X. Gong, Clinicopathological significance of KAI1 expression and epithelial-mesenchymal transition in non-small cell lung cancer. World J. Surg. Oncol. 13, 234 (2015)
Z. Chen, S. Li, K. Huang, Q. Zhang, J. Wang, X. Li, T. Hu, S. Wang, R. Yang, Y. Jia, H. Sun, F. Tang, H. Zhou, J. Shen, D. Ma, The nuclear protein expression levels of SNAI1 and ZEB1 are involved in the progression and lymph node metastasis of cervical cancer via the epithelial-mesenchymal transition pathway. Hum. Pathol. 44(10), 2097–2105 (2013)
A.E. Sayan, T.R. Griffiths, R. Pal, G.J. Browne, A. Ruddick, T. Yagci, R. Edwards, N.J. Mayer, H. Qazi, S. Goyal, S. Fernandez, K. Straatman, G.D. Jones, K.J. Bowman, A. Colquhoun, J.K. Mellon, M. Kriajevska, E. Tulchinsky, SIP1 protein protects cells from DNA damage-induced apoptosis and has independent prognostic value in bladder cancer. Proc. Natl Acad. Sci. USA 106(35), 14884–14889 (2009)
D.A. Budwit-Novotny, K.S. McCarty, E.B. Cox, J.T. Soper, D.G. Mutch, W.T. Creasman, J.L. Flowers, K.S. McCarty Jr., Immunohistochemical analyses of estrogen receptor in endometrial adenocarcinoma using a monoclonal antibody. Cancer Res. 46(10), 5419–5425 (1986)
B. De Craene, G. Berx, Regulatory networks defining EMT during cancer initiation and progression. Nat. Rev. Cancer 13(2), 97–110 (2013)
Z. Yan-Qi, G. Xue-Yan, H. Shuang, C. Yu, G. Fu-Lin, B. Fei-Hu, S. Shi-Ren, W. Xu-Feng, D. Jie, F. Dai-Ming, Expression and significance of TWIST basic helix-loop-helix protein over-expression in gastric cancer. Pathology 39(5), 470–475 (2007)
M. Tania, M.A. Khan, J. Fu, Epithelial to mesenchymal transition inducing transcription factors and metastatic cancer. Tumour Biol. 35(8), 7335–7342 (2014)
S. Lamouille, J. Xu, R. Derynck, Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell. Biol. 15(3), 178–196 (2014)
H. Peinado, D. Olmeda, A. Cano, Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat. Rev. Cancer 7(6), 415–428 (2007)
M.G. Mendez, S. Kojima, R.D. Goldman, Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition. FASEB J. 24(6), 1838–1851 (2010)
T. Else, A.C. Kim, A. Sabolch, V.M. Raymond, A. Kandathil, E.M. Caoili, S. Jolly, B.S. Miller, T.J. Giordano, G.D. Hammer, Adrenocortical carcinoma. Endocr. Rev. 35(2), 282–326 (2014)
S. Mesiano, S.H. Mellon, R.B. Jaffe, Mitogenic action, regulation, and localization of insulin-like growth factors in the human fetal adrenal gland. J. Clin. Endocrinol. Metab. 76(4), 968–976 (1993)
L.F. Leal, L.M. Mermejo, L.Z. Ramalho, C.E. Martinelli Jr., J.A. Yunes, A.L. Seidinger, M.J. Mastellaro, I.A. Cardinalli, S.R. Brandalise, A.C. Moreira, L.G. Tone, C.A. Scrideli, M. Castro, S.R. Antonini, Wnt/beta-catenin pathway deregulation in childhood adrenocortical tumors. J. Clin. Endocrinol. Metab. 96(10), 3106–3114 (2011)
D.C. Gomes, L.F. Leal, L.M. Mermejo, C.A. Scrideli, C.E. Martinelli Jr., M.C. Fragoso, A.C. Latronico, L.G. Tone, S. Tucci, J.A. Yunes, I.A. Cardinalli, M.J. Mastellaro, S.R. Brandalise, F. Ramalho, A.C. Moreira, L.N. Ramalho, M. de Castro, S.R. Antonini, Sonic hedgehog signaling is active in human adrenal cortex development and deregulated in adrenocortical tumors. J. Clin. Endocrinol. Metab. 99(7), E1209–E1216 (2014)
C.L. Ronchi, S. Sbiera, B. Altieri, S. Steinhauer, V. Wild, M. Bekteshi, M. Kroiss, M. Fassnacht, B. Allolio, Notch1 pathway in adrenocortical carcinomas: correlations with clinical outcome. Endocr. Relat. Cancer 22(4), 531–543 (2015)
T. Brabletz, A. Jung, S. Reu, M. Porzner, F. Hlubek, L.A. Kunz-Schughart, R. Knuechel, T. Kirchner, Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc. Natl Acad. Sci. USA 98(18), 10356–10361 (2001)
V. Fendrich, J. Waldmann, F. Esni, A. Ramaswamy, M. Mullendore, M. Buchholz, A. Maitra, G. Feldmann, Snail and Sonic Hedgehog activation in neuroendocrine tumors of the ileum. Endocr. Relat. Cancer 14(3), 865–874 (2007)
K.G. Leong, K. Niessen, I. Kulic, A. Raouf, C. Eaves, I. Pollet, A. Karsan, Jagged1-mediated Notch activation induces epithelial-to-mesenchymal transition through Slug-induced repression of E-cadherin. J. Exp. Med. 204(12), 2935–2948 (2007)
R. Malaguarnera, A. Belfiore, The emerging role of insulin and insulin-like growth factor signaling in cancer stem cells. Front. Endocrinol. 5, 10 (2014)
C. Gicquel, X. Bertagna, V. Gaston, J. Coste, A. Louvel, E. Baudin, J. Bertherat, Y. Chapuis, J.M. Duclos, M. Schlumberger, P.F. Plouin, J.P. Luton, Y. Le Bouc, Molecular markers and long-term recurrences in a large cohort of patients with sporadic adrenocortical tumors. Cancer Res. 61(18), 6762–6767 (2001)
S. Zheng, A.D. Cherniack, N. Dewal, R.A. Moffitt, L. Danilova, B.A. Murray, A.M. Lerario, T. Else, T.A. Knijnenburg, G. Ciriello, S. Kim, G. Assie, O. Morozova, R. Akbani, J. Shih, K.A. Hoadley, T.K. Choueiri, J. Waldmann, O. Mete, A.G. Robertson, H.T. Wu, B.J. Raphael, L. Shao, M. Meyerson, M.J. Demeure, F. Beuschlein, A.J. Gill, S.B. Sidhu, M.Q. Almeida, M.C. Fragoso, L.M. Cope, E. Kebebew, M.A. Habra, T.G. Whitsett, K.J. Bussey, W.E. Rainey, S.L. Asa, J. Bertherat, M. Fassnacht, D.A. Wheeler, G.D. Hammer, T.J. Giordano, R.G. Verhaak, Comprehensive Pan-genomic characterization of adrenocortical carcinoma. Cancer Cell. 29(5), 723–736 (2016)
B. Ragazzon, R. Libe, S. Gaujoux, G. Assie, A. Fratticci, P. Launay, E. Clauser, X. Bertagna, F. Tissier, A. de Reynies, J. Bertherat, Transcriptome analysis reveals that p53 and {beta}-catenin alterations occur in a group of aggressive adrenocortical cancers. Cancer Res. 70(21), 8276–8281 (2010)
F. Tissier, C. Cavard, L. Groussin, K. Perlemoine, G. Fumey, A.M. Hagnere, F. Rene-Corail, E. Jullian, C. Gicquel, X. Bertagna, M.C. Vacher-Lavenu, C. Perret, J. Bertherat, Mutations of beta-catenin in adrenocortical tumors: activation of the Wnt signaling pathway is a frequent event in both benign and malignant adrenocortical tumors. Cancer Res. 65(17), 7622–7627 (2005)
A. Salomon, M. Keramidas, C. Maisin, M. Thomas, Loss of beta-catenin in adrenocortical cancer cells causes growth inhibition and reversal of epithelial-to-mesenchymal transition. Oncotarget 6(13), 11421–11433 (2015)
L. Ozsari, M. Kutahyalioglu, K.M. Elsayes, R.A. Vicens, K. Sircar, T. Jazaerly, S.G. Waguespack, N.L. Busaidy, M.E. Cabanillas, R. Dadu, M.I. Hu, R. Vassilopoulou-Sellin, C. Jimenez, J.E. Lee, M.A. Habra, Preexisting adrenal masses in patients with adrenocortical carcinoma: clinical and radiological factors contributing to delayed diagnosis. Endocrine 51(2), 351–359 (2016)
L. Barzon, M. Chilosi, F. Fallo, G. Martignoni, L. Montagna, G. Palu, M. Boscaro, Molecular analysis of CDKN1C and TP53 in sporadic adrenal tumors. Eur. J. Endocrinol. 145(2), 207–212 (2001)
M. Reincke, M. Karl, W.H. Travis, G. Mastorakos, B. Allolio, H.M. Linehan, G.P. Chrousos, p53 mutations in human adrenocortical neoplasms: immunohistochemical and molecular studies. J. Clin. Endocrinol. Metab. 78(3), 790–794 (1994)
T. Kim, A. Veronese, F. Pichiorri, T.J. Lee, Y.J. Jeon, S. Volinia, P. Pineau, A. Marchio, J. Palatini, S.S. Suh, H. Alder, C.G. Liu, A. Dejean, C.M. Croce, p53 regulates epithelial-mesenchymal transition through microRNAs targeting ZEB1 and ZEB2. J. Exp. Med. 208(5), 875–883 (2011)
E.I. Bimpaki, D. Iliopoulos, A. Moraitis, C.A. Stratakis, MicroRNA signature in massive macronodular adrenocortical disease and implications for adrenocortical tumourigenesis. Clin. Endocrinol. 72(6), 744–751 (2010)
D.R. Szabo, M. Luconi, P.M. Szabo, M. Toth, N. Szucs, J. Horanyi, Z. Nagy, M. Mannelli, A. Patocs, K. Racz, P. Igaz, Analysis of circulating microRNAs in adrenocortical tumors. Lab. Invest. 94(3), 331–339 (2014)
T.J. Giordano, D.G. Thomas, R. Kuick, M. Lizyness, D.E. Misek, A.L. Smith, D. Sanders, R.T. Aljundi, P.G. Gauger, N.W. Thompson, J.M. Taylor, S.M. Hanash, Distinct transcriptional profiles of adrenocortical tumors uncovered by DNA microarray analysis. Am. J. Pathol. 162(2), 521–531 (2003)
J. Waldmann, G. Feldmann, E.P. Slater, P. Langer, M. Buchholz, A. Ramaswamy, W. Saeger, M. Rothmund, V. Fendrich, Expression of the zinc-finger transcription factor Snail in adrenocortical carcinoma is associated with decreased survival. Br. J. Cancer 99(11), 1900–1907 (2008)
A. Comlekci, S. Yener, S. Ertilav, M. Secil, B. Akinci, T. Demir, L. Kebapcilar, F. Bayraktar, S. Yesil, S. Eraslan, Adrenal incidentaloma, clinical, metabolic, follow-up aspects: single centre experience. Endocrine 37(1), 40–46 (2010)
A. Kandathil, K.K. Wong, D.J. Wale, M.C. Zatelli, A.M. Maffione, M.D. Gross, D. Rubello, Metabolic and anatomic characteristics of benign and malignant adrenal masses on positron emission tomography/computed tomography: a review of literature. Endocrine 49(1), 6–26 (2015)
Acknowledgements
The authors are grateful to Dr. Marisa Dreyer Breitenbach for Institutional support. We are also grateful to Priscila Valverde and Maria Theresa Accioly for technical support with immunohistochemistry reactions.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This study was approved by INCA's independent institutional advisory committee in September 24th, 2014 (protocol 33847514.4.0000.5274). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Bulzico, D., Faria, P.S.d., Maia, C.B. et al. Is there a role for epithelial-mesenchymal transition in adrenocortical tumors?. Endocrine 58, 276–288 (2017). https://doi.org/10.1007/s12020-017-1409-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-017-1409-z