Abstract
Purpose
The hypothalamic hormone oxytocin plays a major role in regulation of behavior and body composition. Quality of survival is frequently impaired in childhood craniopharyngioma patients due to sequelae such as behavioral deficits and severe obesity caused by tumor or treatment-related hypothalamic lesions.
Methods
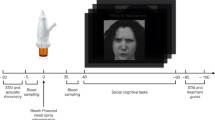
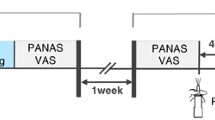
In our pilot cross-sectional study, we analyzed emotion recognition abilities and oxytocin concentrations in saliva and urine before and after single nasal administration of 24 IU oxytocin in 10 craniopharyngioma patients. Four craniopharyngioma presented with grade I lesions (limited to anterior hypothalamic areas) and 6 craniopharyngioma with grade II lesions (involving mammillary bodies and posterior hypothalamic areas). Emotional tasks were assessed before and after administration of oxytocin using the Geneva multimodal emotion portrayals corpus and the Multidimensional Mood Questionnaire.
Results
All patients presented with detectable levels of oxytocin before administration. Nasal administration of oxytocin was well-tolerated and resulted in increased oxytocin concentrations in saliva and urine. After oxytocin administration, craniopharyngioma patients with postsurgical lesions limited to the anterior hypothalamus area showed improvements in emotional identifications compared to craniopharyngioma patients with lesions of anterior and posterior hypothalamic areas. Focusing on correct assignments to positive and negative emotion categories, craniopharyngioma patients improved assignment to negative emotions.
Conclusions
Oxytocin might have positive effects on emotion perception in craniopharyngioma patients with specific lesions of the anterior hypothalamic area. Further studies on larger cohorts are warranted.




Similar content being viewed by others
References
J.E. Blevins, J.M. Ho, Role of oxytocin signaling in the regulation of body weight. Rev. Endocr. Metab. Disord. 14(4), 311–329 (2013). doi:10.1007/s11154-013-9260-x
J.M. Ho, J.E. Blevins, Coming full circle: contributions of central and peripheral oxytocin actions to energy balance. Endocrinology 154(2), 589–596 (2013). doi:10.1210/en.2012-1751
I.D. Neumann, R. Landgraf, Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends. Neurosci. 35(11), 649–659 (2012). doi:10.1016/j.tins.2012.08.004
M. Olff, J.L. Frijling, L.D. Kubzansky, B. Bradley, M.A. Ellenbogen, C. Cardoso, J.A. Bartz, J.R. Yee, M. van Zuiden, The role of oxytocin in social bonding, stress regulation and mental health: an update on the moderating effects of context and interindividual differences. Psychoneuroendocrinology. 38(9), 1883–1894 (2013). doi:10.1016/j.psyneuen.2013.06.019
H.E. Ross, L.J. Young, Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front. Neuroendocrinol. 30(4), 534–547 (2009). doi:10.1016/j.yfrne.2009.05.004
T.R. Insel, The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron 65(6), 768–779 (2010). doi:10.1016/j.neuron.2010.03.005
P.S. Churchland, P. Winkielman, Modulating social behavior with oxytocin: how does it work? What does it mean? Horm. Behav. 61(3), 392–399 (2012). doi:10.1016/j.yhbeh.2011.12.003
A. Romano, B. Tempesta, M.V. Micioni Di Bonaventura, S. Gaetani, From autism to eating disorders and more: the role of oxytocin in neuropsychiatric disorders. Front. Neurosci. 9, 497 (2015). doi:10.3389/fnins.2015.00497
E.A. Lawson, D.A. Marengi, R.L. DeSanti, T.M. Holmes, D.A. Schoenfeld, C.J. Tolley, Oxytocin reduces caloric intake in men. Obesity. (Silver. Spring). 23(5), 950–956 (2015). doi:10.1002/oby.21069
D. Cai, S. Purkayastha, A new horizon: oxytocin as a novel therapeutic option for obesity and diabetes. Drug Discov. Today Dis. Mech. 10(1-2), e63–e68 (2013). doi:10.1016/j.ddmec.2013.05.006
H.L. Muller, Craniopharyngioma. Endocr. Rev. 35(3), 513–543 (2014). doi:10.1210/er.2013-1115
A.S. Sterkenburg, A. Hoffmann, U. Gebhardt, M. Warmuth-Metz, A.M. Daubenbuchel, H.L. Muller, Survival, hypothalamic obesity, and neuropsychological/psychosocial status after childhood-onset craniopharyngioma: newly reported long-term outcomes. Neuro. Oncol. 17(7), 1029–1038 (2015). doi:10.1093/neuonc/nov044
O.M. Dekkers, N.R. Biermasz, J.W. Smit, L.E. Groot, F. Roelfsema, J.A. Romijn, A.M. Pereira, Quality of life in treated adult craniopharyngioma patients. Eur. J. Endocrinol. 154(3), 483–489 (2006). doi:10.1530/eje.1.02114
P. Mortini, M. Losa, G. Pozzobon, R. Barzaghi, M. Riva, S. Acerno, D. Angius, G. Weber, G. Chiumello, M. Giovanelli, Neurosurgical treatment of craniopharyngioma in adults and children: early and long-term results in a large case series. J. Neurosurg. 114(5), 1350–1359 (2011). doi:10.3171/2010.11.JNS10670
H.L. Muller, Consequences of craniopharyngioma surgery in children. J. Clin. Endocrinol. Metab. 96(7), 1981–1991 (2011). doi:10.1210/jc.2011-0174
H.L. Muller, A. Emser, A. Faldum, G. Bruhnken, N. Etavard-Gorris, U. Gebhardt, R. Oeverink, R. Kolb, N. Sorensen, Longitudinal study on growth and body mass index before and after diagnosis of childhood craniopharyngioma. J. Clin. Endocrinol Metab. 89(7), 3298–3305 (2004). doi:10.1210/jc.2003-031751
S.D. Vann, J.P. Aggleton, The mammillary bodies: two memory systems in one? Nat. Rev. Neurosci. 5(1), 35–44 (2004). doi:10.1038/nrn1299
J. Ozyurt, H.L. Muller, C.M. Thiel, A systematic review of cognitive performance in patients with childhood craniopharyngioma. J. Neurooncol. 125(1), 9–21 (2015). doi:10.1007/s11060-015-1885-z
H.L. Muller, Childhood craniopharyngioma. Recent advances in diagnosis, treatment and follow-up. Horm. Res. 69(4), 193–202 (2008). doi:10.1159/000113019
A. Pierre-Kahn, C. Recassens, G. Pinto, C. Thalassinos, S. Chokron, J.C. Soubervielle, R. Brauner, M. Zerah, C. Sainte Rose, Social and psycho-intellectual outcome following radical removal of craniopharyngiomas in childhood. A prospective series. Child. Nerv. Syst. 21(8-9), 817–824 (2005). doi:10.1007/s00381-005-1205-6
G. Zada, N. Kintz, M. Pulido, L. Amezcua, Prevalence of neurobehavioral, social, and emotional dysfunction in patients treated for childhood craniopharyngioma: a systematic literature review. PLoS ONE 8(11), e76562 (2013). doi:10.1371/journal.pone.0076562
A. Meyer-Lindenberg, G. Domes, P. Kirsch, M. Heinrichs, Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat. Rev. Neurosci. 12(9), 524–538 (2011). doi:10.1038/nrn3044
J. Ozyurt, A. Lorenzen, U. Gebhardt, M. Warmuth-Metz, H.L. Muller, C.M. Thiel, Remote effects of hypothalamic lesions in the prefrontal cortex of craniopharygioma patients. Neurobiol. Learn. Mem. 111, 71–80 (2014). doi:10.1016/j.nlm.2014.03.007
N. Cook, J. Miller, J. Hart, Parent observed neuro-behavioral and pro-social improvements with oxytocin following surgical resection of craniopharyngioma. J. Pediatr. Endocrinol. Metab. (2016). doi:10.1515/jpem-2015-0445
E. Hollander, J. Bartz, W. Chaplin, A. Phillips, J. Sumner, L. Soorya, E. Anagnostou, S. Wasserman, Oxytocin increases retention of social cognition in autism. Biol. Psychiatry 61(4), 498–503 (2007). doi:10.1016/j.biopsych.2006.05.030
I.F. Lin, M. Kashino, H. Ohta, T. Yamada, M. Tani, H. Watanabe, C. Kanai, T. Ohno, Y. Takayama, A. Iwanami, N. Kato, The effect of intranasal oxytocin versus placebo treatment on the autonomic responses to human sounds in autism: a single-blind, randomized, placebo-controlled, crossover design study. Mol. Autism. 5(1), 20 (2014). doi:10.1186/2040-2392-5-20
M.F. Rolland-Cachera, T.J. Cole, M. Sempe, J. Tichet, C. Rossignol, A. Charraud, Body Mass Index variations: centiles from birth to 87 years. Eur. J. Clin. Nutr. 45(1), 13–21 (1991)
H.L. Muller, U. Gebhardt, C. Teske, A. Faldum, I. Zwiener, M. Warmuth-Metz, T. Pietsch, F. Pohl, N. Sorensen, G. Calaminus, Post-operative hypothalamic lesions and obesity in childhood craniopharyngioma: results of the multinational prospective trial KRANIOPHARYNGEOM 2000 after 3-year follow-up. Eur. J. Endocrinol. 165(1), 17–24 (2011). doi:10.1530/EJE-11-0158
H.L. Muller, U. Gebhardt, A. Faldum, M. Warmuth-Metz, T. Pietsch, F. Pohl, G. Calaminus, N. Sorensen, Xanthogranuloma, Rathke’s cyst, and childhood craniopharyngioma: results of prospective multinational studies of children and adolescents with rare sellar malformations. J. Clin. Endocrinol. Metab. 97(11), 3935–3943 (2012). doi:10.1210/jc.2012-2069
R. Landgraf, Plasma oxytocin concentrations in man after different routes of administration of synthetic oxytocin. Exp. Clin. Endocrinol. 85(2), 245–248 (1985). doi:10.1055/s-0029-1210444
I. Neumann, M. Ludwig, M. Engelmann, Q.J. Pittman, R. Landgraf, Simultaneous microdialysis in blood and brain: oxytocin and vasopressin release in response to central and peripheral osmotic stimulation and suckling in the rat. Neuroendocrinology 58(6), 637–645 (1993)
T. Banziger, M. Mortillaro, K.R. Scherer, Introducing the Geneva Multimodal expression corpus for experimental research on emotion perception. Emotion 12(5), 1161–1179 (2012). doi:10.1037/a0025827
R. Steyer, O. Schwenkmezger, P. Notz, M. Eid. Der Mehrdimensionale Befindlicheitsfragebogen (MDBF). Hogrefe, Göttingen, (1997)
J.E. Wolff, E. Daumling, A. Dirksen, A. Dabrock, M. Hartmann, H. Jurgens, Munster Heidelberg Abilities Scale--a measuring instrument for global comparison of illness sequelae. Klin. Padiatr. 208(5), 294–298 (1996). doi:10.1055/s-2008-1046486
C.S. Carter, Oxytocin and sexual behavior. Neurosci. Biobehav. Rev. 16(2), 131–144 (1992)
O.J. Bosch, I.D. Neumann, Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: from central release to sites of action. Horm. Behav. 61(3), 293–303 (2012). doi:10.1016/j.yhbeh.2011.11.002
J.R. Williams, T.R. Insel, C.R. Harbaugh, C.S. Carter, Oxytocin administered centrally facilitates formation of a partner preference in female prairie voles (Microtus ochrogaster). J. Neuroendocrinol. 6(3), 247–250 (1994)
T.R. Insel, R.D. Fernald, How the brain processes social information: searching for the social brain. Ann. Rev. Neurosci. 27, 697–722 (2004). doi:10.1146/annurev.neuro.27.070203.144148
A. Benelli, A. Bertolini, R. Poggioli, B. Menozzi, R. Basaglia, R. Arletti, Polymodal dose-response curve for oxytocin in the social recognition test. Neuropeptides 28(4), 251–255 (1995)
M. Engelmann, K. Ebner, C.T. Wotjak, R. Landgraf, Endogenous oxytocin is involved in short-term olfactory memory in female rats. Behav. Brain Res. 90(1), 89–94 (1998)
K. Lancaster, C.S. Carter, H. Pournajafi-Nazarloo, T. Karaoli, T.S. Lillard, A. Jack, J.M. Davis, J.P. Morris, J.J. Connelly, Plasma oxytocin explains individual differences in neural substrates of social perception. Front. Hum. Neurosci. 9, 132 (2015). doi:10.3389/fnhum.2015.00132
M. Lukas, I. Toth, A.H. Veenema, I.D. Neumann, Oxytocin mediates rodent social memory within the lateral septum and the medial amygdala depending on the relevance of the social stimulus: male juvenile versus female adult conspecifics. Psychoneuroendocrinology 38(6), 916–926 (2013). doi:10.1016/j.psyneuen.2012.09.018
J.E. Ferguson 2nd, B.H. Head, F.H. Frank, M.L. Frank, J.S. Singer, T. Stefos, G. Mari, Misoprostol versus low-dose oxytocin for cervical ripening: a prospective, randomized, double-masked trial. Am. J. Obstet. Gynecol. 187(2), 273–279 (2002). discussion 279-280
J.A. Bartz, J. Zaki, N. Bolger, K.N. Ochsner, Social effects of oxytocin in humans: context and person matter. Trends Cogn. Sci. 15(7), 301–309 (2011). doi:10.1016/j.tics.2011.05.002
G. Domes, M. Heinrichs, A. Michel, C. Berger, S.C. Herpertz, Oxytocin improves “mind-reading” in humans. Biol. Psychiatry 61(6), 731–733 (2007). doi:10.1016/j.biopsych.2006.07.015
M. Tauber, C. Mantoulan, P. Copet, J. Jauregui, G. Demeer, G. Diene, B. Roge, V. Laurier, V. Ehlinger, C. Arnaud, C. Molinas, D. Thuilleaux, Oxytocin may be useful to increase trust in others and decrease disruptive behaviours in patients with Prader-Willi syndrome: a randomised placebo-controlled trial in 24 patients. Orphanet. J. Rare. Dis. 6, 47 (2011). doi:10.1186/1750-1172-6-47
R.J. Kuppens, S.H. Donze, A.C. Hokken-Koelega, Promising effects of oxytocin on social and food-related behavior in young children with Prader-Willi Syndrome: a randomized, double-blind, controlled crossover trial. Clin. Endocrinol. (2016). doi:10.1111/cen.13169
S.L. Einfeld, E. Smith, I.S. McGregor, K. Steinbeck, J. Taffe, L.J. Rice, S.K. Horstead, N. Rogers, M.A. Hodge, A.J. Guastella, A double-blind randomized controlled trial of oxytocin nasal spray in Prader Willi syndrome. Am. J. Med. Genet. A 164A(9), 2232–2239 (2014). doi:10.1002/ajmg.a.36653
L. Johnson, A.M. Manzardo, J.L. Miller, D.J. Driscoll, M.G. Butler, Elevated plasma oxytocin levels in children with Prader-Willi syndrome compared with healthy unrelated siblings. Am. J. Med. Genet. A 170(3), 594–601 (2016). doi:10.1002/ajmg.a.37488
A.M. Daubenbuchel, A. Hoffmann, M. Eveslage, J. Ozyurt, K. Lohle, J. Reichel, C.M. Thiel, H. Martens, V. Geenen, H.L. Muller, Oxytocin in survivors of childhood-onset craniopharyngioma. Endocrine 54(2), 524–531 (2016). doi:10.1007/s12020-016-1084-5
M. Mercedes Perez-Rodriguez, K. Mahon, M. Russo, A.K. Ungar, K.E. Burdick, Oxytocin and social cognition in affective and psychotic disorders. Eur. Neuropsychopharmacol. 25(2), 265–282 (2015). doi:10.1016/j.euroneuro.2014.07.012
P. Kirsch, C. Esslinger, Q. Chen, D. Mier, S. Lis, S. Siddhanti, H. Gruppe, V.S. Mattay, B. Gallhofer, A. Meyer-Lindenberg, Oxytocin modulates neural circuitry for social cognition and fear in humans. J. Neurosci. 25(49), 11489–11493 (2005). doi:10.1523/JNEUROSCI.3984-05.2005
P. Popik, J. Vetulani, J.M. van Ree, Low doses of oxytocin facilitate social recognition in rats. Psychopharmacology (Berl). 106(1), 71–74 (1992)
H.G. Bauer, Endocrine and other clinical manifestations of hypothalamic disease; a survey of 60 cases, with autopsies. J. Clin. Endocrinol. Metab. 14(1), 13–31 (1954). doi:10.1210/jcem-14-1-13
M. Fischer-Shofty, S.G. Shamay-Tsoory, Y. Levkovitz, Characterization of the effects of oxytocin on fear recognition in patients with schizophrenia and in healthy controls. Front. Neurosci. 7, 127 (2013). doi:10.3389/fnins.2013.00127
M.E. McCullough, P.S. Churchland, A.J. Mendez, Problems with measuring peripheral oxytocin: can the data on oxytocin and human behavior be trusted? Neurosci. Biobehav. Rev. 37(8), 1485–1492 (2013). doi:10.1016/j.neubiorev.2013.04.018
G. Lippi, G.C. Guidi, C. Mattiuzzi, M. Plebani, Preanalytical variability: the dark side of the moon in laboratory testing. Clin. Chem. Lab. Med. 44(4), 358–365 (2006). doi:10.1515/CCLM.2006.073
Acknowledgements
This study was funded by a grant (Forschungspool) of the European Medical School, Oldenburg, Germany, and a grant (DKS2014.13) of the German Childhood Cancer Foundation, Bonn, Germany. H. Müller is supported by the German Childhood Cancer Foundation, Bonn, Germany.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interest.
Ethical Approval
All procedures performed in our study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the local standing committee on ethical practice as an individual medical treatment in ten cases and written patient consent was obtained in all cases.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Hoffmann, A., Özyurt, J., Lohle, K. et al. First experiences with neuropsychological effects of oxytocin administration in childhood-onset craniopharyngioma. Endocrine 56, 175–185 (2017). https://doi.org/10.1007/s12020-017-1257-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-017-1257-x