Abstract
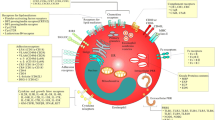
The eosinophil is a fully delineated granulocyte that disseminates throughout the bloodstream to end-organs after complete maturation in the bone marrow. While the presence of eosinophils is not uncommon even in healthy individuals, these granulocytes play a central role in inflammation and allergic processes. Normally appearing in smaller numbers, higher levels of eosinophils in the peripheral blood or certain tissues typically signal a pathologic process. Eosinophils confer a beneficial effect on the host by enhancing immunity against molds and viruses. However, tissue-specific elevation of eosinophils, particularly in the respiratory system, can cause a variety of short-term symptoms and may lead to long-term sequelae. Eosinophils often play a role in more commonly encountered disease processes, such as asthma and allergic responses in the upper respiratory tract. They are also integral in the pathology of less common diseases including eosinophilic pneumonia, allergic bronchopulmonary aspergillosis, hypersensitivity pneumonitis, and drug reaction with eosinophilia and systemic symptoms. They can be seen in neoplastic disorders or occupational exposures as well. The involvement of eosinophils in pulmonary disease processes can affect the method of diagnosis and the selection of treatment modalities. By analyzing the complex interaction between the eosinophil and its environment, which includes signaling molecules and tissues, different therapies have been discovered and created in order to target disease processes at a cellular level. Innovative treatments such as mepolizumab and benralizumab will be discussed. The purpose of this article is to further explore the topic of eosinophilic presence, activity, and pathology in the respiratory tract, as well as discuss current and future treatment options through a detailed literature review.



Similar content being viewed by others
References
Wardlaw AJ, Brightling C, Green R, Woltmann G, Pavord I (2000) Eosinophils in asthma and other allergic diseases. Br Med Bull 56:985–1003
Scott KA, Wardlaw AJ (2006) Eosinophilic airway disorders. Semin Respir Crit Care Med 27:128–133
Adkinson NF, Bochner BS, Busse WW et al (2009) Middleton’s allergy: principles and practice, 7th edn. Mosby/Elsevier, Philadelphia
Adkinson NF, Bochner BS, Burks AW et al (2014) Middleton’s allergy: principles and practice, 8th edn. Elsevier/Saunders, Philadelphia
Stone KD, Prussin C, Metcalfe DD (2010) IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol 125:S73–S80
Percopo CM, Dyer KD, Ochkur SI et al (2014) Activated mouse eosinophils protect against lethal respiratory virus infection. Blood 123:743–752
Lilly LM, Scopel M, Nelson MP, Burg AR, Dunaway CW, Steele C (2014) Eosinophil deficiency compromises lung defense against Aspergillus fumigatus. Infect Immun 82:1315–1325
Wechsler ME (2007) Pulmonary eosinophilic syndromes. Immunol Allergy Clin N Am 27:477–492
Cottin V, Cordier JF (2012) Eosinophilic lung diseases. Immunol Allergy Clin N Am 32:557–586
Jeong YJ, Kim KI, Seo IJ et al (2007) Eosinophilic lung diseases: a clinical, radiologic, and pathologic overview. Radiographics Rev Publ Radiol Soc North Am Inc 27:617–637, discussion 637-619
Ying S, Meng Q, Zeibecoglou K et al (1999) Eosinophil chemotactic chemokines (eotaxin, eotaxin-2, RANTES, monocyte chemoattractant protein-3 (MCP-3), and MCP-4), and C-C chemokine receptor 3 expression in bronchial biopsies from atopic and nonatopic (Intrinsic) asthmatics. J Immunol 163:6321–6329
Peterson MW, Monick M, Hunninghake GW (1987) Prognostic role of eosinophils in pulmonary fibrosis. Chest 92:51–56
Henderson WR, Chi EY, Klebanoff SJ (1980) Eosinophil peroxidase-induced mast cell secretion. J Exp Med 152:265–279
Dechatelet LR, Migler RA, Shirley PS, Bass DA, McCall CE (1978) Enzymes of oxidative metabolism in the human eosinophil. Proceedings of the Society for Experimental Biology and Medicine. Soc Exp Biol Med 158:537–541
Rudd RM, Haslam PL, Turner-Warwick M (1981) Cryptogenic fibrosing alveolitis. Relationships of pulmonary physiology and bronchoalveolar lavage to response to treatment and prognosis. Am Rev Respir Dis 124:1–8
Frigas E, Loegering DA, Gleich GJ (1980) Cytotoxic effects of the guinea pig eosinophil major basic protein on tracheal epithelium. Lab Investig J Tech Methods Pathol 42:35–43
Davis WB, Fells GA, Sun XH, Gadek JE, Venet A, Crystal RG (1984) Eosinophil-mediated injury to lung parenchymal cells and interstitial matrix. A possible role for eosinophils in chronic inflammatory disorders of the lower respiratory tract. J Clin Investig 74:269–278
Weller PF (2014) Approach to the patient with unexplained eosinophilia
Daimon T, Johkoh T, Sumikawa H et al (2008) Acute eosinophilic pneumonia: thin-section CT findings in 29 patients. Eur J Radiol 65:462–467
Sohn JW (2013) Acute eosinophilic pneumonia. Tuberc Respir Dis 74:51–55
Rom WN, Weiden M, Garcia R et al (2002) Acute eosinophilic pneumonia in a New York City firefighter exposed to World Trade Center dust. Am J Respir Crit Care Med 166:797–800
Philit F, Etienne-Mastroianni B, Parrot A, Guerin C, Robert D, Cordier JF (2002) Idiopathic acute eosinophilic pneumonia: a study of 22 patients. Am J Respir Crit Care Med 166:1235–1239
Rose DM, Hrncir DE (2013) Primary eosinophilic lung diseases. Allergy Asthma Proc Off J Reg State Allergy Soc 34:19–25
Janz DR, O’Neal HR Jr, Ely EW (2009) Acute eosinophilic pneumonia: a case report and review of the literature. Crit Care Med 37:1470–1474
Cottin V, Cordier JF (2005) Eosinophilic pneumonias. Allergy 60:841–857
Carrington CB, Addington WW, Goff AM et al (1969) Chronic eosinophilic pneumonia. N Engl J Med 280:787–798
Ge Y, Han X, Liu X (2013) Chronic eosinophilic pneumonia: a case report and review of the literature. Open J Internal Med 3:121–125
Akuthota P, Weller PF (2012) Eosinophilic pneumonias. Clin Microbiol Rev 25:649–660
Okubo Y, Horie S, Hachiya T et al (1998) Predominant implication of IL-5 in acute eosinophilic pneumonia: comparison with chronic eosinophilic pneumonia. Int Arch Allergy Immunol 116:76–80
Jhun BW, Kim SJ, Kim K, Lee JE, Hong DJ (2014) Clinical implications of correlation between peripheral eosinophil count and serum levels of IL-5 and tryptase in acute eosinophilic pneumonia. Respir Med
Obayashi Y, Yamadori I, Fujita J, Yoshinouchi T, Ueda N, Takahara J (1997) The role of neutrophils in the pathogenesis of idiopathic pulmonary fibrosis. Chest 112:1338–1343
Prasad R, Gupta N, Singh A, Gupta P (2015) Diagnosis of idiopathic pulmonary fibrosis: current issues. Intractable Rare Diseases Res 4:65–69
Iwai K, Mori T, Yamada N, Yamaguchi M, Hosoda Y (1994) Idiopathic pulmonary fibrosis. Epidemiologic approaches to occupational exposure. Am J Respir Crit Care Med 150:670–675
Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G (2006) Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 174:810–816
Kroegel C, Foerster M, Grahmann PR, Braun R (1998) Eosinophil priming and migration in idiopathic pulmonary fibrosis. Eur Respir J 11:999–1001
Gioffredi A, Maritati F, Oliva E, Buzio C (2014) Eosinophilic granulomatosis with polyangiitis: an overview. Front Immunol 5:549
Klion A (2009) Hypereosinophilic syndrome: current approach to diagnosis and treatment. Annu Rev Med 60:293–306
Ogbogu PU, Bochner BS, Butterfield JH et al (2009), Hypereosinophilic syndromes: a multicenter, retrospective analysis of clinical characteristics and response to therapy. J Allergy Clin Immunol. 124, 1319-1325
Fernandez Perez ER, Olson AL, Frankel SK (2011) Eosinophilic lung diseases. Med Clin North Am 95:1163–1187
Mullarkey MF, Hill JS, Webb DR (1980) Allergic and nonallergic rhinitis: their characterization with attention to the meaning of nasal eosinophilia. J Allergy Clin Immunol 65:122–126
Bachert C (2004) Persistent rhinitis—allergic or nonallergic? Allergy 59(Suppl 76):11–15, discussion 15
Moneret-Vautrin DA, Hsieh V, Wayoff M, Guyot JL, Mouton C, Maria Y (1990) Nonallergic rhinitis with eosinophilia syndrome a precursor of the triad: nasal polyposis, intrinsic asthma, and intolerance to aspirin. Annals of allergy 64:513–518
Leone C, Teodoro C, Pelucchi A et al (1997) Bronchial responsiveness and airway inflammation in patients with nonallergic rhinitis with eosinophilia syndrome. J Allergy Clin Immunol 100:775–780
Ellis AK, Keith PK (2006) Nonallergic rhinitis with eosinophilia syndrome. Current Allergy Asthma Rep 6:215–220
Fang CH, Mady LJ, Mirani NM, Baredes S, Eloy JA (2014) Sinonasal eosinophilic angiocentric fibrosis: a systematic review. Int Forum Allergy Rhinology 4:745–752
Nguyen DB, Alex JC, Calhoun B (2004) Eosinophilic angiocentric fibrosis in a patient with nasal obstruction. Ear Nose Throat J 83:183–184, 186
Onder S, Sungur A (2004) Eosinophilic angiocentric fibrosis: an unusual entity of the sinonasal tract. Arch Pathol Lab Med 128:90–91
Jain R, Robblee JV, O’Sullivan-Mejia E et al (2008) Sinonasal eosinophilic angiocentric fibrosis: a report of four cases and review of literature. Head Neck Pathol 2:309–315
Lotvall J, Akdis CA, Bacharier LB et al (2011) Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol 127:355–360
Lemanske RF Jr, Busse WW (2010) Asthma: clinical expression and molecular mechanisms. J Allergy Clin Immunol 125:S95–S102
National Asthma E, Prevention P (2007) Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol 120:S94–S138
Keglowich L, Roth M, Philippova M et al (2013) Bronchial Smooth muscle cells of asthmatics promote angiogenesis through elevated secretion of CXC-Chemokines (ENA-78, GRO-alpha, and IL-8). PLoS One 8, e81494
Agache I, Akdis C, Jutel M, Virchow JC (2012) Untangling asthma phenotypes and endotypes. Allergy 67:835–846
Miranda C, Busacker A, Balzar S, Trudeau J, Wenzel SE (2004) Distinguishing severe asthma phenotypes: role of age at onset and eosinophilic inflammation. J Allergy Clin Immunol 113:101–108
Haldar P, Pavord ID, Shaw DE et al (2008) Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med 178:218–224
Green RH, Brightling CE, McKenna S et al (2002) Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet 360:1715–1721
Al-Samri MT, Benedetti A, Prefontaine D et al (2010) Variability of sputum inflammatory cells in asthmatic patients receiving corticosteroid therapy: A prospective study using multiple samples. J Allergy Clin Immunol 125(1161–1163), e1164
Wang F, He XY, Baines KJ et al (2011) Different inflammatory phenotypes in adults and children with acute asthma. Eur Respir J 38:567–574
Walford HH, Doherty TA (2014) Diagnosis and management of eosinophilic asthma: a US perspective. J Asthma Allergy 7:53–65
Yoo Y (2013) Phenotypes and endotypes of severe asthma in children. Korean J Pediatr 56:191–195
Campo P, Rodriguez F, Sanchez-Garcia S et al (2013) Phenotypes and endotypes of uncontrolled severe asthma: new treatments. J Investig Allergol Clin Immunol 23:76–88, quiz 71 p follow 88
Xie M, Wenzel SE (2013) A global perspective in asthma: from phenotype to endotype. Chin Med J 126:166–174
Wenzel S (2012) Severe asthma: from characteristics to phenotypes to endotypes. Clin Exp Allergy J Br Soc Allergy Clin Immunol 42:650–658
Hamelmann E, Gelfand EW (2001) IL-5-induced airway eosinophilia—the key to asthma? Immunol Rev 179:182–191
Pace E, Ferraro M, Siena L et al (2008) Cigarette smoke increases Toll-like receptor 4 and modifies lipopolysaccharide-mediated responses in airway epithelial cells. Immunology 124:401–411
Monick MM, Yarovinsky TO, Powers LS et al (2003) Respiratory syncytial virus up-regulates TLR4 and sensitizes airway epithelial cells to endotoxin. J Biol Chem 278:53035–53044
Galowitz S, Chang C (2014) Immunobiology of Critical Pediatric Asthma. Clin Rev Allergy Immunol
Muzio M, Polentarutti N, Bosisio D, Manoj Kumar PP, Mantovani A (2000) Toll-like receptor family and signalling pathway. Biochem Soc Trans 28:563–566
Dabbagh K, Dahl ME, Stepick-Biek P, Lewis DB (2002) Toll-like receptor 4 is required for optimal development of Th2 immune responses: role of dendritic cells. J Immunol 168:4524–4530
Saenz SA, Siracusa MC, Perrigoue JG et al (2010) IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature 464:1362–1366
Neill DR, Wong SH, Bellosi A et al (2010) Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 464:1367–1370
Fort MM, Cheung J, Yen D et al (2001) IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity 15:985–995
Schneider E, Petit-Bertron AF, Bricard R et al (2009) IL-33 activates unprimed murine basophils directly in vitro and induces their in vivo expansion indirectly by promoting hematopoietic growth factor production. J Immunol 183:3591–3597
Zhou B, Comeau MR, De Smedt T et al (2005) Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol 6:1047–1053
Fang C, Siew LQ, Corrigan CJ, Ying S (2010) The role of thymic stromal lymphopoietin in allergic inflammation and chronic obstructive pulmonary disease. Arch Immunol Ther Exp 58:81–90
Trivedi SG, Lloyd CM (2007) Eosinophils in the pathogenesis of allergic airways disease. Cell Mol Life Sci CMLS 64:1269–1289
Jeffery PK (2000) Comparison of the structural and inflammatory features of COPD and asthma. Giles F Filley Lect Chest 117:251S–260S
Qiu Y, Zhu J, Bandi V, Guntupalli KK, Jeffery PK (2007) Bronchial mucosal inflammation and upregulation of CXC chemoattractants and receptors in severe exacerbations of asthma. Thorax 62:475–482
Fahy JV, Kim KW, Liu J, Boushey HA (1995) Prominent neutrophilic inflammation in sputum from subjects with asthma exacerbation. J Allergy Clin Immunol 95:843–852
Sur S, Crotty TB, Kephart GM et al (1993) Sudden-onset fatal asthma. A distinct entity with few eosinophils and relatively more neutrophils in the airway submucosa? Am Rev Respir Dis 148:713–719
Qiu Y, Zhu J, Bandi V et al (2003) Biopsy neutrophilia, neutrophil chemokine and receptor gene expression in severe exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 168:968–975
Imaizumi T, Albertine KH, Jicha DL, McIntyre TM, Prescott SM, Zimmerman GA (1997) Human endothelial cells synthesize ENA-78: relationship to IL-8 and to signaling of PMN adhesion. Am J Respir Cell Mol Biol 17:181–192
Wuyts A, Proost P, Lenaerts JP, Ben-Baruch A, Van Damme J, Wang JM (1998) Differential usage of the CXC chemokine receptors 1 and 2 by interleukin-8, granulocyte chemotactic protein-2 and epithelial-cell-derived neutrophil attractant-78. Eur J Biochem FEBS 255:67–73
Moore WC, Meyers DA, Wenzel SE et al (2010) Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med 181:315–323
Fitzpatrick AM, Teague WG, Meyers DA et al (2011) Heterogeneity of severe asthma in childhood: confirmation by cluster analysis of children in the national institutes of health/national heart, lung, and blood institute severe asthma research program. J Allergy Clin Immunol 127:382–389, e381-313
Jarjour NN, Erzurum SC, Bleecker ER et al (2012) Severe asthma: lessons learned from the national heart, lung, and blood institute severe asthma research program. Am J Respir Crit Care Med 185:356–362
Bossley CJ, Fleming L, Gupta A et al (2012) Pediatric severe asthma is characterized by eosinophilia and remodeling without T(H)2 cytokines. J Allergy Clin Immunol 129:974–982, e913
Silkoff PE, Lent AM, Busacker AA et al (2005) Exhaled nitric oxide identifies the persistent eosinophilic phenotype in severe refractory asthma. J Allergy Clin Immunol 116:1249–1255
Saglani S, Lloyd CM (2014) Eosinophils in the pathogenesis of paediatric severe asthma. Curr Opin Allergy Clin Immunol 14:143–148
Lemiere C, Ernst P, Olivenstein R et al (2006) Airway inflammation assessed by invasive and noninvasive means in severe asthma: eosinophilic and noneosinophilic phenotypes. J Allergy Clin Immunol 118:1033–1039
Saglani S, Malmstrom K, Pelkonen AS et al (2005) Airway remodeling and inflammation in symptomatic infants with reversible airflow obstruction. Am J Respir Crit Care Med 171:722–727
Marguet C, Bocquel N, Benichou J et al (2008) Neutrophil but not eosinophil inflammation is related to the severity of a first acute epidemic bronchiolitis in young infants. Pediatr Allergy Immunol Off Publ Eur Soc Pediatr Allergy Immunol 19:157–165
de Blic J, Tillie-Leblond I, Tonnel AB, Jaubert F, Scheinmann P, Gosset P (2004) Difficult asthma in children: an analysis of airway inflammation. J Allergy Clin Immunol 113:94–100
Arshad SH, Raza A, Lau L et al (2014) Pathophysiological characterization of asthma transitions across adolescence. Respir Res 15:153
Sly PD, Boner AL, Bjorksten B et al (2008) Early identification of atopy in the prediction of persistent asthma in children. Lancet 372:1100–1106
Stern DA, Morgan WJ, Halonen M, Wright AL, Martinez FD (2008) Wheezing and bronchial hyper-responsiveness in early childhood as predictors of newly diagnosed asthma in early adulthood: a longitudinal birth-cohort study. Lancet 372:1058–1064
Gibson PG, Simpson JL, Saltos N (2001) Heterogeneity of airway inflammation in persistent asthma : evidence of neutrophilic inflammation and increased sputum interleukin-8. Chest 119:1329–1336
Gibson PG (2009) Inflammatory phenotypes in adult asthma: clinical applications. Clin Respir J 3:198–206
Jenkins HA, Cherniack R, Szefler SJ, Covar R, Gelfand EW, Spahn JD (2003) A comparison of the clinical characteristics of children and adults with severe asthma. Chest 124:1318–1324
Nizankowska E, Soja J, Pinis G et al (1995) Treatment of steroid-dependent bronchial asthma with cyclosporin. Eur Respir J 8:1091–1099
Presta LG, Lahr SJ, Shields RL et al (1993) Humanization of an antibody directed against IgE. J Immunol 151:2623–2632
Schulman ES (2001) Development of a monoclonal anti-immunoglobulin E antibody (omalizumab) for the treatment of allergic respiratory disorders. Am J Respir Crit Care Med 164:S6–S11
Erin EM, Leaker BR, Nicholson GC et al (2006) The effects of a monoclonal antibody directed against tumor necrosis factor-alpha in asthma. Am J Respir Crit Care Med 174:753–762
Gauvreau GM, Pageau R, Seguin R et al (2011) Dose-response effects of TPI ASM8 in asthmatics after allergen. Allergy 66:1242–1248
Simpson JL, Powell H, Boyle MJ, Scott RJ, Gibson PG (2008) Clarithromycin targets neutrophilic airway inflammation in refractory asthma. Am J Respir Crit Care Med 177:148–155
Ortega HG, Liu MC, Pavord ID et al (2014) Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med 371:1198–1207
Bel EH, Wenzel SE, Thompson PJ et al (2014) Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med 371:1189–1197
Laviolette M, Gossage DL, Gauvreau G et al (2013) Effects of benralizumab on airway eosinophils in asthmatic patients with sputum eosinophilia. J Allergy Clin Immunol 132(1086–1096), e1085
Castro M, Wenzel SE, Bleecker ER et al. (2014) Benralizumab, an anti-interleukin 5 receptor alpha monoclonal antibody, versus placebo for uncontrolled eosinophilic asthma: a phase 2b randomised dose-ranging study. Lancet Respir Med
Fitzpatrick AM, Gaston BM, Erzurum SC, Teague WG, National Institutes of Health/National Heart and P. Blood Institute Severe Asthma Research (2006) Features of severe asthma in school-age children: atopy and increased exhaled nitric oxide. J Allergy Clin Immunol 118:1218–1225
Covar RA, Spahn JD, Martin RJ et al (2004) Safety and application of induced sputum analysis in childhood asthma. J Allergy Clin Immunol 114:575–582
Wedes SH, Wu W, Comhair SA et al (2011) Urinary bromotyrosine measures asthma control and predicts asthma exacerbations in children. J Pediatr 159(248–255), e241
Selman M (2004) Hypersensitivity pneumonitis: a multifaceted deceiving disorder. Clin Chest Med 25(531–547):vi
Lacasse Y, Cormier Y (2006) Hypersensitivity pneumonitis. Orphanet J Rare Dis 1:25
Monkare S, Ikonen M, Haahtela T (1985) Radiologic findings in farmer’s lung. Prognosis Correlation Lung Funct Chest 87:460–466
Cormier Y, Belanger J, LeBlanc P, Laviolette M (1986) Bronchoalveolar lavage in farmers’ lung disease: diagnostic and physiological significance. Br J Ind Med 43:401–405
Lacasse Y, Selman M, Costabel U et al (2003) Clinical diagnosis of hypersensitivity pneumonitis. Am J Respir Crit Care Med 168:952–958
Monkare S (1983) Influence of corticosteroid treatment on the course of farmer’s lung. Eur J Respir Dis 64:283–293
Bochenek G, Nizankowska-Mogilnicka E (2013) Aspirin-exacerbated respiratory disease: clinical disease and diagnosis. Immunol Allergy Clin N Am 33:147–161
Burnett T, Katial R, Alam R (2013) Mechanisms of aspirin desensitization. Immunol Allergy Clin N Am 33:223–236
Choi JH, Kim MA, Park HS (2014) An update on the pathogenesis of the upper airways in aspirin-exacerbated respiratory disease. Curr Opin Allergy Clin Immunol 14:1–6
Saltzstein SL, Ackerman LV (1959) Lymphadenopathy induced by anticonvulsant drugs and mimicking clinically pathologically malignant lymphomas. Cancer 12:164–182
Camous X, Calbo S, Picard D, Musette P (2012) Drug Reaction with eosinophilia and systemic symptoms: an update on pathogenesis. Curr Opin Immunol 24:730–735
Bocquet H, Bagot M, Roujeau JC (1996) Drug-induced pseudolymphoma and drug hypersensitivity syndrome (Drug Rash with Eosinophilia and Systemic Symptoms: DRESS). Semin Cutaneous Med Surg 15:250–257
Tetart F, Picard D, Janela B, Joly P, Musette P (2013) Prolonged Evolution of Drug Reaction With Eosinophilia and Systemic Symptoms: Clinical, Virologic, and Biological Features. JAMA Dermatol
Peyriere H, Dereure O, Breton H et al (2006) Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatology 155:422–428
Favrolt N, Bonniaud P, Collet E et al (2007) Severe drug rash with eosinophilia and systemic symptoms after treatment with minocycline. Rev Mal Respir 24:892–895
Husain Z, Reddy BY, Schwartz RA (2013) DRESS syndrome: Part I. Clinical perspectives. J Am Acad Dermatol 68:693, e691-614; quiz 706-698
Kano Y, Shiohara T (2009) The variable clinical picture of drug-induced hypersensitivity syndrome/drug rash with eosinophilia and systemic symptoms in relation to the eliciting drug. Immunol Allergy Clin N Am 29:481–501
Tohyama M, Hashimoto K, Yasukawa M et al (2007) Association of human herpesvirus 6 reactivation with the flaring and severity of drug-induced hypersensitivity syndrome. Br J Dermatol 157:934–940
Heiner DC, Sears JW, Kniker WT (1962) Multiple precipitins to cow’s milk in chronic respiratory disease. A syndrome including poor growth, gastrointestinal symptoms, evidence of allergy, iron deficiency anemia, and pulmonary hemosiderosis. Am J Dis Child 103:634–654
Moissidis I, Chaidaroon D, Vichyanond P, Bahna SL (2005) Milk-induced pulmonary disease in infants (Heiner syndrome). Pediatric Allergy Immunol Off Publ Eur Soc Pediatr Allergy Immunol 16:545–552
Tan J, Bernstein JA (2014) Occupational asthma: an overview. Curr Allergy Asthma Rep 14:431
Lummus ZL, Wisnewski AV, Bernstein DI (2011) Pathogenesis and disease mechanisms of occupational asthma. Immunol Allergy Clin N Am 31(699–716):vi
Malo JL, Vandenplas O (2011) Definitions and classification of work-related asthma. Immunol Allergy Clin N Am 31:645–662, v
Enriquez A, Fernandez C, Jimenez A, Seoane E, Alcorta AR, Rodriguez J (2011) Occupational asthma induced by Mucor species contaminating esparto fibers. J Investig Allergol Clin Immunol 21:251–252
Agarwal R, Chakrabarti A, Shah A et al (2013) Allergic bronchopulmonary aspergillosis: review of literature and proposal of new diagnostic and classification criteria. Clin Exp Allergy J Br Soc Allergy Clin Immunol 43:850–873
Gibson PG (2006) Allergic bronchopulmonary aspergillosis. Semin Respir Crit Care Med 27:185–191
Wark PA, Hensley MJ, Saltos N et al (2003) Anti-inflammatory effect of itraconazole in stable allergic bronchopulmonary aspergillosis: a randomized controlled trial. J Allergy Clin Immunol 111:952–957
Moss RB (2012) The use of biological agents for the treatment of fungal asthma and allergic bronchopulmonary aspergillosis. Ann N Y Acad Sci 1272:49–57
Zirbes JM, Milla CE (2008) Steroid-sparing effect of omalizumab for allergic bronchopulmonary aspergillosis and cystic fibrosis. Pediatr Pulmonol 43:607–610
Gupta D (2011) Allergic Bronchopulmonary Aspergillosis (ABPA): Relevance of Absolute Eosinophil Count (AEC) in Peripheral Blood. Chest 140
Denning DW, O’Driscoll BR, Hogaboam CM, Bowyer P, Niven RM (2006) The link between fungi and severe asthma: a summary of the evidence. Eur Respir J 27:615–626
Denning DW, O’Driscoll BR, Powell G et al (2009) Randomized controlled trial of oral antifungal treatment for severe asthma with fungal sensitization: The Fungal Asthma Sensitization Trial (FAST) study. Am J Respir Crit Care Med 179:11–18
Klion AD, Nutman TB (2004) The role of eosinophils in host defense against helminth parasites. J Allergy Clin Immunol 113:30–37
Craig JM, Scott AL (2014) Helminths in the lungs. Parasite Immunol 36:463–474
Keiser PB, Nutman TB (2004) Strongyloides stercoralis in the Immunocompromised Population. Clin Microbiol Rev 17:208–217
Shin MH, Lee YA, Min DY (2009) Eosinophil-mediated tissue inflammatory responses in helminth infection. Korean J Parasitol 47(Suppl):S125–S131
Pandit R, Scholnik A, Wulfekuhler L, Dimitrov N (2007) Non-small-cell lung cancer associated with excessive eosinophilia and secretion of interleukin-5 as a paraneoplastic syndrome. Am J Hematol 82:234–237
Slungaard A, Ascensao J, Zanjani E, Jacob HS (1983) Pulmonary carcinoma with eosinophilia. Demonstration of a tumor-derived eosinophilopoietic factor. N Engl J Med 309:778–781
Wilson F, Tefferi A (2005) Acute lymphocytic leukemia with eosinophilia: two case reports and a literature review. Leukemia & Lymphoma 46:1045–1050
Furuta K, Taki M, Murase H, Nakagawa A, Nishiyama H, Nohgawa M (2011) [A case of eosinophilic pneumonia which occurred after bone marrow transplantation for acute myeloid leukemia]. Journal of the. Jpn Respir Soc 49:20–24
Trojan A, Meier R, Licht A, Taverna C (2002) Eosinophilic pneumonia after administration of fludarabine for the treatment of non-Hodgkin’s lymphoma. Ann Hematol 81:535–537
Verstraeten AS, De Weerdt A, van Den Eynden G, Van Marck E, Snoeckx A, Jorens PG (2011) Excessive eosinophilia as paraneoplastic syndrome in a patient with non-small-cell lung carcinoma: a case report and review of the literature. Acta Clin Belg 66:293–297
Stevens DA, Moss RB, Kurup VP et al (2003) Allergic bronchopulmonary aspergillosis in cystic fibrosis--state of the art: cystic fibrosis foundation consensus conference. Clin Infect Dis Off Publ Infect Dis Soc Am 37(Suppl 3):S225–S264
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Drs. Eng and DeFelice have disclosed no financial relationships relevant to this article. This commentary does not contain a discussion of an unapproved/investigative use of a commercial product or device.
Rights and permissions
About this article
Cite this article
Eng, S.S., DeFelice, M.L. The Role and Immunobiology of Eosinophils in the Respiratory System: a Comprehensive Review. Clinic Rev Allerg Immunol 50, 140–158 (2016). https://doi.org/10.1007/s12016-015-8526-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-015-8526-3