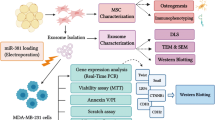
Abstract
Exosomes, nano-sized cell-derived vesicles, have been employed as non-synthetic carriers of various pharmaceutics in numerous studies. As higher expression levels of miR-142-3p and miR-150 in breast cancer stem cells (BCSCs) are associated with their clonogenic and tumorigenic capabilities, the present study aims to exploit the mesenchymal stem cells-derived exosomes (MSCs-Exo) to deliver LNA-antimiR-142-3p into MCF7-derived cancer stem-like cells to suppress expression levels of miR-142-3p and miR-150 in order to reduce clonogenicity and tumorigenicity. Our results indicated that the MSCs-Exo can efficiently deliver the LNA-antimiR-142-3p to breast cancer stem-like cells to reduce the miR-142-3p and miR-150 expression levels. Furthermore, the inhibition of the oncomiRs with the delivery of LNA-antimiR-142-3p resulted in a significant reduction of clone-formation and tumor-initiating abilities of the MCF7-derived cancer stem-like cells. In conclusion, we showed that MSCs-derived exosomes could be used as a feasible nanovehicles to deliver RNA-based therapeutics into BCSCs to improve the cancer treatment.
Highlights
-
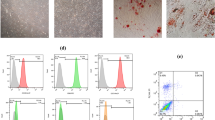
Exosomes secreted by bone marrow-derived mesenchymal stem cells efficiently transfer the LNA-antimiR-142-3p to breast cancer stem cells.
-
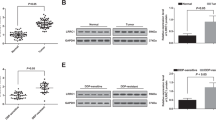
Exosomes-mediated delivery of LNA-antimiR-142-3p to the breast cancer stem cells leads to downregulation of miR-142-3p and miR-150 and the overexpression of target genes.
-
Delivery of LNA-antimiR-142-3p by the exosomes reduces the colony formation capability of breast cancer stem cells in vitro.
-
Inhibition of miR-142-3p and miR-150 by the LNA-antimiR-142-3p loaded exosomes reduces the tumorigenicity of breast cancer stem cells in vivo.









Similar content being viewed by others
References
Andaloussi, S. E., Mäger, I., Breakefield, X. O., & Wood, M. J. (2013). Extracellular vesicles: Biology and emerging therapeutic opportunities. Nature Reviews Drug Discovery, 12, 347.
Vlassov, A. V., Magdaleno, S., Setterquist, R., & Conrad, R. (2012). Exosomes: Current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochimica et Biophysica Acta (BBA)-General Subjects, 1820, 940–948.
Morishita, M., Takahashi, Y., Nishikawa, M., & Takakura, Y. (2017). Pharmacokinetics of exosomes—An important factor for elucidating the biological roles of exosomes and for the development of exosome-based therapeutics. Journal of Pharmaceutical Sciences, 106, 2265–2269.
Colombo, M., Raposo, G., & Théry, C. (2014). Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annual Review of Cell and Developmental Biology, 30, 255–289.
Johnsen, K. B., Gudbergsson, J. M., Skov, M. N., Pilgaard, L., Moos, T., & Duroux, M. (2014). A comprehensive overview of exosomes as drug delivery vehicles—Endogenous nanocarriers for targeted cancer therapy. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer, 1846, 75–87.
Wicha, M. S., Liu, S., & Dontu, G. (2006). Cancer stem cells: An old idea—A paradigm shift. Cancer Research, 66, 1883–1890.
Al-Hajj, M., Wicha, M. S., Benito-Hernandez, A., Morrison, S. J., & Clarke, M. F. (2003). Prospective identification of tumorigenic breast cancer cells. Proceedings of the National Academy of Sciences, 100, 3983–3988.
Setoguchi, T., Taga, T., & Kondo, T. (2004). Cancer stem cells persist in many cancer cell lines. Cell Cycle, 3, 412–413.
Shimono, Y., Zabala, M., Cho, R. W., et al. (2009). Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell, 138, 592–603.
Isobe, T., Hisamori, S., Hogan, D. J., et al. (2014). miR-142 regulates the tumorigenicity of human breast cancer stem cells through the canonical WNT signaling pathway. Elife, 3, e01977.
Singh, M. S., & Peer, D. (2016). SiRNA delivery: Current trends and future perspectives. Therapeutic Delivery, 7, 51–53.
Shtam, T. A., Kovalev, R. A., Varfolomeeva, E. Y., Makarov, E. M., Kil, Y. V., & Filatov, M. V. (2013). Exosomes are natural carriers of exogenous siRNA to human cells in vitro. Cell Communication and Signaling, 11, 88.
Umezu, T., Ohyashiki, K., Kuroda, M., & Ohyashiki, J. (2013). Leukemia cell to endothelial cell communication via exosomal miRNAs. Oncogene, 32, 2747.
Zhu, L., Qu, X.-H., Sun, Y.-L., Qian, Y.-M., & Zhao, X.-H. (2014). Novel method for extracting exosomes of hepatocellular carcinoma cells. World Journal Of Gastroenterology: WJG, 20, 6651.
Momen-Heravi, F., Bala, S., Bukong, T., & Szabo, G. (2014). Exosome-mediated delivery of functionally active miRNA-155 inhibitor to macrophages. Nanomedicine: Nanotechnology, Biology and Medicine, 10, 1517–1527.
Naseri, Z., Oskuee, R. K., Jaafari, M. R., & Moghadam, M. F. (2018). Exosome-mediated delivery of functionally active mirna-142-3p inhibitor reduces tumorigenicity of breast cancer in vitro and in vivo. International Journal of Nanomedicine, 13, 7727.
Duan, J.-j., Qiu, W., Xu, S.-l., et al. (2013). Strategies for isolating and enriching cancer stem cells: Well begun is half done. Stem Cells and Development, 22, 2221–2239.
Charafe-Jauffret, E., Ginestier, C., Iovino, F., Wicinski, J., Cervera, N., Finetti, P., Hur, M. H., Diebel, M. E., Monville, F., Dutcher, J., Brown, M., Viens, P., Xerri, L., Bertucci, F., Stassi, G., Dontu, G., Birnbaum, D., & Wicha, M. S. (2009). Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Research, 69, 1302–1313.
Cairo, S., Wang, Y., de Reyniès, A., et al. (2010). Stem cell-like micro-RNA signature driven by Myc in aggressive liver cancer. Proceedings of the National Academy of Sciences, 107, 20471–20476.
Hwang-Verslues, W., Chang, P., Wei, P., et al. (2011). miR-495 is upregulated by E12/E47 in breast cancer stem cells, and promotes oncogenesis and hypoxia resistance via downregulation of E-cadherin and REDD1. Oncogene, 30, 2463.
Wang, Y., Yu, Y., Tsuyada, A., et al. (2011). Transforming growth factor-β regulates the sphere-initiating stem cell-like feature in breast cancer through miRNA-181 and ATM. Oncogene, 30, 1470.
Zhou, A., Diao, L., Xu, H., et al. (2012). β-Catenin/LEF1 transactivates the microRNA-371-373 cluster that modulates the Wnt/β-catenin-signaling pathway. Oncogene, 31, 2968.
Wellner, U., Schubert, J., Burk, U. C., et al. (2009). The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nature Cell Biology, 11, 1487.
Huang, S., Chen, Y., Wu, W., et al. (2013). miR-150 promotes human breast cancer growth and malignant behavior by targeting the pro-apoptotic purinergic P2X7 receptor. PloS One, 8, e80707.
Hu, W., Ye, Y., Zhang, W., Wang, J., Chen, A., & Guo, F. (2013). miR-142-3p promotes osteoblast differentiation by modulating Wnt signaling. Molecular Medicine Reports, 7, 689–693.
Nayerossadat, N., Maedeh, T., & Ali, P. A. (2012). Viral and nonviral delivery systems for gene delivery. Advanced Biomedical Research, 1, 27.
Scholz, C., & Wagner, E. (2012). Therapeutic plasmid DNA versus siRNA delivery: Common and different tasks for synthetic carriers. Journal of Controlled Release, 161, 554–565.
Lakhal, S., & Wood, M. J. (2011). Exosome nanotechnology: An emerging paradigm shift in drug delivery: Exploitation of exosome nanovesicles for systemic in vivo delivery of RNAi heralds new horizons for drug delivery across biological barriers. Bioessays, 33, 737–741.
Alvarez-Erviti, L., Seow, Y., Yin, H., Betts, C., Lakhal, S., & Wood, M. J. (2011). Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nature Biotechnology, 29, 341–345.
Lässer, C., Eldh, M., & Lötvall, J. (2012). Isolation and characterization of RNA-containing exosomes. JoVE (Journal of Visualized Experiments), e3037.
Munoz, J. L., Bliss, S. A., Greco, S. J., Ramkissoon, S. H., Ligon, K. L., & Rameshwar, P. (2013). Delivery of functional anti-miR-9 by mesenchymal stem cell–derived exosomes to glioblastoma multiforme cells conferred chemosensitivity. Molecular Therapy-Nucleic Acids, 2, e126.
Ohno, S.-i., Takanashi, M., Sudo, K., et al. (2013). Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Molecular Therapy, 21, 185–191.
Kim, S. H., Bianco, N. R., Shufesky, W. J., Morelli, A. E., & Robbins, P. D. (2007). Effective treatment of inflammatory disease models with exosomes derived from dendritic cells genetically modified to express IL-4. The Journal of Immunology, 179, 2242–2249.
Lee, J.-K., Park, S.-R., Jung, B.-K., et al. (2013). Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLoS One, 8, e84256.
McLellan, A. (2009). Exosome release by primary B cells. Critical Reviews™ in Immunology, 29, 203–217.
Takahashi, Y., Nishikawa, M., Shinotsuka, H., Matsui, Y., Ohara, S., Imai, T., & Takakura, Y. (2013). Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. Journal of Biotechnology, 165, 77–84.
Greco, S. J., & Rameshwar, P. (2012). Mesenchymal stem cells in drug/gene delivery: Implications for cell therapy. Therapeutic Delivery, 3, 997–1004.
Sundaram, B., Herbert, F. J., & Kumar, S. (2017). Human mesenchymal stem cell (hMSC)-derived exosomes/exosome mimetics as a potential novel therapeutic tool for regenerative medicine. Regenerative Medicine: Laboratory to Clinic: Springer, 81–97.
Sharif, S., Ghahremani, M., & Soleimani, M. (2018). Delivery of exogenous miR-124 to glioblastoma multiform cells by Wharton’s jelly mesenchymal stem cells decreases cell proliferation and migration, and confers chemosensitivity. Stem Cell Reviews and Reports, 14, 236–246.
Katakowski, M., Buller, B., Zheng, X., Lu, Y., Rogers, T., Osobamiro, O., Shu, W., Jiang, F., & Chopp, M. (2013). Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Letters, 335, 201–204.
Acknowledgments
We are grateful to our colleagues and PhD students, for help and support during this work, in the Department of Medical Biotechnology, Faculty of Medical Sciences, Tarbiat Modares University, Tehran. We also thank Mrs. Karen Fascioli for editing the manuscript.
Funding
This research was funded by Mashhad University of Medical Sciences and Tarbiat Modares University, Tehran (NO.931289); and a grant from cancer research center of cancer institute of Iran (Shams cancer charity, Grant No: 37604–202–01-97). The funding sources played no decisive role in collection, analysis, the interpretation of the data, as well as decision to submit for publication.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 15.3 kb)
Rights and permissions
About this article
Cite this article
naseri, Z., Oskuee, R.K., forouzandeh-moghadam, M. et al. Delivery of LNA-antimiR-142-3p by Mesenchymal Stem Cells-Derived Exosomes to Breast Cancer Stem Cells Reduces Tumorigenicity. Stem Cell Rev and Rep 16, 541–556 (2020). https://doi.org/10.1007/s12015-019-09944-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-019-09944-w