Abstract
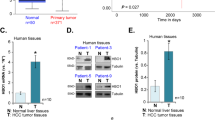
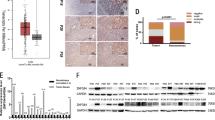
We previously reported that the abnormal BTG2 expression was related to genesis/development of hepatocellular carcinoma (HCC). The aim of this study was to evaluate the BTG2 expression in HCC compared with p53, cyclin D1, and cyclin E. For this purpose, modified diethylnitrosamine (DEN)-induced primary HCC rat model was established. Target proteins and mRNAs were measured by western blot and RT-PCR/northern blot, respectively. In rat liver, expression of BTG2 and other proteins was determined by western blot, and BTG2 mRNA in HCC/normal tissues was detected by high-flux tissue microarray (TMA) and in situ hybridization (ISH). BTG2 mRNA/protein expression was increased in fetal liver, 7701, and LO2 cell lines but decreased in HepG2 cells. BTG2/p53 were expressed early after DEN treatment, peaked at 5 weeks and decreased gradually thereafter. Cyclin-D1/Cyclin-E expression increased significantly with the tumor progression. BTG2 mRNA was expressed in 71.19% HCC by ISH and correlated with differentiation. Expression of p53/cyclin D1/cyclin E was positive in 82.35/94.12/76.47% BTG2 mRNA-negative tissues, respectively. BTG2 protein expression was lost in 32.2% (19/59) HCC tissues, and the mRNA/protein expression correlated significantly with the increasing tumor grade (P < 0.05). In conclusion, BTG2 expression is commonly impaired in HCC which may be a factor involved in deregulation of cyclin-D1/cyclin-E expression during hepatocarcinogenesis.



Similar content being viewed by others
References
Sukhatme, V. P., Kartha, S., Toback, F. G., Taub, R., Hoover, R. G., & Tsai-Morris, C. H. (1987). A novel early growth response gene rapidly induced by fibroblast, epithelial cell and lymphocyte mitogens. Oncogene Research, 1, 343–355.
Tirone, F. (2001). The gene PC3TIS21/BTG2, prototype member of the PC3/BTG/TOB family: Regulator in control of cell growth, differentiation, and DNA repair? Journal of Cellular Physiology, 187, 155–165.
Guehenneux, F., Duret, L., Callanan, M. B., Bouhas, R., Hayette, S., Berthet, C., et al. (1997). Cloning of the mouse BTG3 gene and definition of a new gene family (the BTG family) involved in the negative control of the cell cycle. Leukemia, 11, 370–375.
Bradbury, A., Possenti, R., Shooter, E. M., & Tirone, F. (1991). Molecular cloning of PC3, a putatively secreted protein whose mRNA is induced by nerve growth factor and depolarization. Proceedings of the National Academy of Sciences of the United States of America, 88, 3353–3357.
Fletcher, B. S., Lim, R. W., Varnum, B. C., Kujubu, D. A., Koski, R. A., & Herschman, H. R. (1991). Structure and expression of TIS21, a primary response gene induced by growth factors and tumor promoters. Journal of Biological Chemistry, 266, 14511–14518.
Duriez, C., Falette, N., Audoynaud, C., Moyret-Lalle, C., Bensaad, K., Courtois, S., et al. (2002). The human BTG2/TIS21/PC3 gene: Genomic structure, transcriptional regulation and evaluation as a candidate tumor suppressor gene. Gene, 282, 207–214.
Rouault, J. P., Falette, N., Guéhenneux, F., Guillot, C., Rimokh, R., Wang, Q., et al. (1996). Identification of BTG2, an antiproliferative p53-dependent component of the DNA damage cellular response pathway. Nature Genetics, 14, 482–486.
Puisieux, A., & Magaud, J. P. (1999). Mechanisms of BTG2 activity, a transcriptional target of p53: Evidences and hypothesis. Bull Cancer, 86, 358–364.
DelSal, G., Loda, M., & Pagano, M. (1996). Cell cycle and cancer: Critical events at the G1-restriction point. Critical Reviews in Oncogenesis, 7, 127–142.
Sherr, C. J. (1996). Cancer cell cycles. Science, 274, 1672–1677.
Guardavaccaro, D., Corrente, G., Covone, F., Micheli, L., D’Agnano, I., Starace, G., et al. (2000). Arrest of G1-S progression by the p53-inducible gene PC3 is Rb dependent and relies on the inhibition of cyclin D1 transcription. Molecular and Cellular Biology, 20, 1797–1815.
Lim, I. K., Lee, M. S., Ryu, M. S., Park, T. J., Fujiki, H., Eguchi, H., et al. (1998). Overexpression of TIS21 in 293 cells induces growth inhibition by down-regulating the cyclin E and cyclin-dependent kinase 4 proteins. Molecular Carcinogenesis, 23, 25–35.
Lin, W. J., Gary, J. D., Yang, M. C., Clarke, S., & Herschman, H. R. (1996). The mammalian immediate early TIS21 protein and the leukemia associated BTG1 protein interact with a protein-arginine N-methyltransferase. Journal of Biological Chemistry, 271, 15034–15044.
Rouault, J. P., Prevot, D., Berthet, C., Birot, A. M., Billaud, M., Magaud, J. P., et al. (1998). Interaction of BTG1 and p53-regulated BTG2 gene products with mCaf1, the murine homolog of a component of the yeast CCR4 transcriptional regulatory complex. Journal of Biological Chemistry, 273, 22563–22569.
Prevot, D., Voeltzel, T., Birot, A. M., Morel, A. P., Rostan, M. C., Magaud, J. P., et al. (2000). The leukemia-associated protein Btg1 and the p53-regulated protein BTG2 interact with the homeoprotein Hoxb9 and enhance its transcriptional activation. Journal of Biological Chemistry, 275, 147–153.
Kawakubo, H., Carey, J. L., Brachtel, E., Gupta, V., Green, J. E., Walden, P. D., et al. (2004). Expression of the NF-kappaB-responsive gene BTG2 is aberrantly regulated in breast cancer. Oncogene, 23, 8310–8319.
Kawakubo, H., Carey, J. L., Brachtel, E., Hayashida, T., Yeo, G., Kish, J., et al. (2006). Loss of B-cell translocation gene-2 in estrogen receptor positive breast carcinoma is associated with tumor grade and overexpression of cyclin D1 protein. Cancer Research, 66, 7075–7082.
Ficazzola, M. A., Fraiman, M., Gitlin, J., Woo, K., Melamed, J., Rubin, M. A., et al. (2001). Antiproliferative B cell translocation gene 2 protein is down-regulated post-transcriptionally as an early event in prostate carcinogenesis. Carcinogenesis, 22, 1271–1279.
Struckmann, K., Schraml, P., Simon, R., Elmenhorst, K., Mirlacher, M., Kononen, J., et al. (2004). Impaired expression of the cell cycle regulator BTG2 is common in clear cell renal cell carcinoma. Cancer Research, 64, 1632–1638.
Melamed, J., Kernizan, S., & Walden, P. D. (2002). Expression of B-cell translocation gene 2 protein in normal human tissues. Tissue and Cell, 34, 28–32.
Xu, W. X., Wang, S. Y., Wang, G., Wei, H., He, F., & Yang, X. (2000). Identification and characterization of differentially expressed genes in the early response phase during liver regeneration. Biochemical and Biophysical Research Communications, 278, 318–325.
Zhang, Z. M., Wang, G., Chen, C., Yang, Z. X., Jin, F., San, J. L., et al. (2009). Rapid induction of PC3/BTG2 gene by hepatopoietin or partial hepatectomy and its mRNA expression in hepatocellular carcinoma. Hepatobiliary and Pancreatic Diseases International, 8, 288–293.
Ge, W., En-ren, L., Lu, H., Wang, J., Leng, E. R., & Fang, D. C. (2002). Rapid Induction of mRNAs for PC3 genes by hepatopoietin and partial hepatectomy. Chinese Journal of Hepatology, 10, 256–259.
Sundarajan, M., Gupta, S., & Rao, K. V. K. (2002). Overexpression of cyclin D1 is associated with the decondensation of chromatin during DEN-induced hepatocarcinogenesis. Cell Biology International, 26, 699–706.
Fukumasu, H., Avanzo, J. L., Heidor, R., Silva, T. C., Atroch, A., Moreno, F. S., et al. (2006). Protective effects of guarana (Paullinia cupana Mart. var. sorbilis) against DEN-induced DNA damage on mouse liver. Food and Chemical Toxicology, 44, 862–867.
Kallioniemi, O. P., Wagner, U., Kononen, J., & Sauter, G. (2001). Tissue microarray technology for high-throughput molecular profiling of cancer. Human Molecular Genetics, 10, 657–662.
Onuma, K., Dabbs, D. J., & Bhargava, R. (2008). Mammaglobin expression in the female genital tract: Immunohistochemical analysis in benign and neoplastic endocervix and endometrium. International Journal of Gynecological Pathology, 27, 418–425.
Park, T. J., Kim, J. Y., Paul Oh, S., Kang, S. Y., Kim, B. W., Wang, H. J., et al. (2008). TIS21 negatively regulates hepatocarcinogenesis by disruption of cyclin B1-Forkhead box M1 regulation loop. Hepatology, 47, 1533–1543.
Matsuda, S., Rouault, J., Magaud, J., & Berthet, C. (2001). In search of a function for the TIS21/PC3/BTG1/TOB family. FEBS Letters, 497, 67–72.
Liu, M., Wu, H., Liu, T., Li, Y., Wang, F., Wan, H., et al. (2009). Regulation of the cell cycle gene, BTG2, by miR-21 in human laryngeal carcinoma. Cell Research, 19, 828–837.
LI, Q., Wang, G., & Zhang, Z. M. (2009). The relationship between microRNA-18 and BTG2 in the carcinogenesis of hepatocellular carcinoma [Article in Chinese]. Zhonghua Gan Zang Bing Za Zhi, 17, 42–45.
Acknowledgments
We thank the Chinese National Natural Science Foundation (Grants #30973457, #30901764) and the Academic Foundation for Authors of National Excellent Doctoral Dissertation of China (Award # 200261) for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Zhimin Zhang and Chuan Chen contributed equally to this study as co-first authors.
Rights and permissions
About this article
Cite this article
Zhang, Z., Chen, C., Wang, G. et al. Aberrant Expression of the p53-Inducible Antiproliferative Gene BTG2 in Hepatocellular Carcinoma is Associated with Overexpression of the Cell Cycle-Related Proteins. Cell Biochem Biophys 61, 83–91 (2011). https://doi.org/10.1007/s12013-011-9164-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-011-9164-x