Abstract
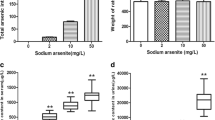
Although studies have shown that arsenic exposure can induce apoptosis in a variety of cells, the exact molecular mechanism of chronic arsenicosis remains unclear. Based on our previous study on human serum, the present study was to determine whether pigment epithelium-derived factor (PEDF) plays a role in the damage induced by chronic arsenic exposure in a rat model and to explore the possible signaling pathway involved. Thirty male Wistar rats were randomly divided into three groups and the arsenite doses administered were 0, 10, and 50 mg/L, respectively. The experiment lasted for 6 months. Our results showed that level of arsenic increased significantly in serum, liver, brain, and kidney in arsenic-exposed groups. It was indicated that PEDF protein was widely distributed in the cytoplasm of various types of cells in liver, brain, and kidney. PEDF protein level was only changed when the arsenite dose reached 50 mg/L in liver and brain, whereas it was not changed in the kidney. In order to investigate the possible mechanism of PEDF-exerted damages upon arsenite exposure, apoptosis in liver and brain was assessed. The proportion of apoptotic cells gradually increased with increasing arsenic administration. The ratio of Bax/Bcl-2 in the high arsenic group (50 mg/L) was significantly higher than that in the control group. Therefore, we thought PEDF played a role in cell apoptosis of liver and brain which induced by sodium arsenite exposure, and the results also demonstrated that Bax and Bcl-2 might be two key targets in the action of PEDF.






Similar content being viewed by others
References
Tondel M, Rahman M, Magnuson A et al (1999) The relationship of arsenic levels in drinking water and the prevalence rate of skin lesions in Bangladesh. Environ Health Perspect 107:727–729
Yu G, Sun DJ, Zheng Y (2007) Health effects of exposure to natural arsenic in groundwater and coal in China: an overview of occurrence. Environ Health Perspect 115:636–642
Sun G, Li FJ, Li GX et al (1998) The exploration for the mechanism of arsenic on kidney toxicity in mice. Chin J Endemiol 17:284–286
Sun G (2007) The progress on the mechanism research of drinking-type endemic arsenism. J Med Res 36:2–4
Bashir S, Sharma Y, Irshad M et al (2006) Arsenic induced apoptosis in rat liver following repeated 60 days exposure. Toxicology 217:63–70
Namgung U, Xia Z (2001) Arsenic induces apoptosis in rat cerebellar neurons via activation of JNK3 and p38 MAP kinases. Toxicol Appl Pharmacol 174:130–138
Qu W, Bortner CD, Sakurai T et al (2002) Acquisition of apoptotic resistance in arsenic-induced malignant transformation: role of the JNK signal transduction pathway. Carcinogenesis 23:151–159
Shi Y, Wei Y, Qu S et al (2010) Arsenic induces apoptosis of human umbilical vein endothelial cells through mitochondrial pathways. Cardiovasc Toxicol 10:153–160
Zhi F, Wang XG (2011) Arsenite-induced apoptosis is prevented by selenitein A375 cell line. Biol Trace Elem Res 140:7–17
Liu J, Michael P (2008) Liver is a target of arsenic carcinogenesis. Toxicol Sci 105:24–32
Tombran-Tink J, Chader GG, Johnson LV et al (1991) PEDF: pigment epithelium-derived factor with potent neuronal differentiative activity. Exp Eye Res 53:411–414
Ek E, Dass CR, Chong PF (2006) PEDF: a potential molecular therapeutic target with multiple anticancer activities. Trends Mol Med 12:497–502
Zhao LJ, Gao YH, Li YY et al (2012) Differential expression of serum proteins in chronic arsenic exposed population. Chin J Endemiol 31:7–12
Liu J, Liu Y, Goyer RA (2000) Metallothionein-I/II null mice are more sensitive than wild-type mice to the hepatotoxic and nephrotoxic effects of chronic oral or injected inorganic arsenicals. Toxicol Sci 55:460–467
Majhi C, Khan S, Leo MD et al (2011) Effects of acetaminophen on reactive oxygen species and nitric oxide redox signaling in kidney of arsenic-exposed rats. Food Chem Toxicol 49:974–982
Huang M, Choi SJ, Kim DW et al (2009) Risk assessment of low-level cadmium and arsenic on the kidney. J Toxicol Environ Health A72:1493–1498
Wang J, Zhang W, Sun DJ et al (2010) Analysis of neuroglobin mRNA expression in rat Brain due to arsenite-induced oxidative stress. Environ Toxicol. doi:10.1002/tox.20664
Abe R, Shimizu T, Yamagishi S et al (2004) Over expression of pigment epithelium-derived factor decreases angiogenesis and inhibits the growth of human malignant melanoma cells in vivo. Am J Pathol 164:1225–1232
Amano S, Yamagishi S, Inagaki Y et al (2005) Pigment epitheliumderived factor inhibits oxidative stress-induced apoptosis and dysfunction of cultured retinal pericytes. Microvasc Res 69:45–55
Garcia M, Fernandez-Garcia NI, Rivas V et al (2004) Inhibition of xenografted human melanoma growth and prevention of metastasis development by dual antiangiogenic/antitumor activities of pigment epithelium-derived factor. Cancer Res 64:5632–5642
Kawaguchi T, Yamagishi S, Itou M et al (2010) Pigment epithelium-derived factor inhibits lysosomal degradation of Bcl-xL and apoptosis in HepG2 cells. Am J Pathol 176:168–176
Yamagishi S, Inagaki Y, Amano S et al (2002) Pigment epithelium derived factor protects cultured retinal pericytes from advanced glycation end product-induced injury through its anti-oxidative properties. Biochem Biophys Res Commun 296:877–882
Zhang T, Guan M, Xu C et al (2007) Pigment epithelium-derived factor inhibits glioma cell growth in vitro and in vivo. Life Sci 81:1256–1263
Shimizu S, Ide T, Yanagida T et al (2000) Electrophysiological study of a novel large pore formed by Bax and the voltage-dependent anion channel that is permeable to cytochrome c. J Biol Chem 275:12321–12325
Mikhailov V, Mikhailova M, Degenhardt K et al (2003) Association of Bax and Bak homo-oligomers in mitochondria Bax requirement for Bak reorganization and cytochrome C release. J Biol Chem 278:5367–5376
Acknowledgments
This research was supported by the 11th Five-Year Planning Project of Science and Technology Supporting (project no. 2006BAI06B04), Ministry of Science and Technology, China.
Conflict of interest
There are no conflicts of interest for any of the authors.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Wei Zhang, Hongqi Feng and Yanhui Gao contributed equally to this paper.
Rights and permissions
About this article
Cite this article
Zhang, W., Feng, H., Gao, Y. et al. Role of Pigment Epithelium-Derived Factor (PEDF) in Arsenic-Induced Cell Apoptosis of Liver and Brain in a Rat Model. Biol Trace Elem Res 151, 269–276 (2013). https://doi.org/10.1007/s12011-012-9558-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-012-9558-7