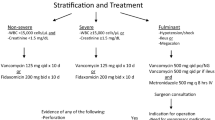
Abstract
Purpose of review
This article will review current management strategies for severe and fulminant Clostridioides difficile infection (CDI).
Recent findings
Clostridioides difficile is the most common nosocomial cause of infectious diarrhea. With the rise of hypervirulent strains of CDI, almost 8% of patients hospitalized with CDI are afflicted with severe CDI (SCDI) or fulminant CDI (FCDI). A significant proportion of these patients do not respond to recommended anti-CDI antibiotic therapy such as oral vancomycin and fidaxomicin. Current recommendations suggest that patients with refractory CDI should proceed to colectomy or diverting loop ileostomy with colonic lavage. However, both of these surgical interventions result in high rates of post-surgical mortality approaching 30%. Fecal microbiota transplantation (FMT) is a promising therapy that is recommended in recurrent CDI. Recent studies have found that FMT can safely produce cure rates between 70 and 90% in patients with SCDI and FCDI, while significantly decreasing rates of CDI-related mortality and colectomy. A patient population likely to benefit the most from FMT is elderly patients due to their increased risk for CDI, treatment failure, and high comorbidity burden that may preclude surgical intervention.
Summary
FMT should be considered in patients with SCDI or FCDI particularly when traditional anti-CDI antibiotics are ineffective.
Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:• Of importance ••Of major importance
Gould CV, McDonald LC, Lessa FC. Current status of Clostridium difficile infection epidemiology. Clin Infect Dis. 2012;55(suppl_2):S65–70.
Rao K, Micic D, Natarajan M, Winters S, Kiel MJ, Walk ST, et al. Clostridium difficile ribotype 027: relationship to age, detectability of toxins A or B in stool with rapid testing, severe infection, and mortality. Clin Infect Dis. 2015;61(2):233–41.
Vindigni SM, Surawicz CM. C. difficile infection: changing epidemiology and management paradigms. Clin Transl Gastroenterol. 2015;6(7):e99–9.
McDonald LC, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):e1–e48.
Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald L, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431–55.
Surawicz CM, Brandt LJ, Binion DG, Ananthakrishnan AN, Curry SR, Gilligan PH, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108(4):478–98 quiz 499.
Miller M, et al. Health care-associated Clostridium difficile infection in Canada: patient age and infecting strain type are highly predictive of severe outcome and mortality. Clin Infect Dis. 2010;50(2):194–201.
Lessa FC, et al. NAP1 strain type predicts outcomes from Clostridium difficile infection. Clin Infect Dis. 2014;58(10):1394–400.
Henrich TJ, et al. Clinical risk factors for severe Clostridium difficile-associated disease. Emerg Infect Dis. 2009;15(3):415–22.
Chitnis AS, Holzbauer SM, Belflower RM, Winston LG, Bamberg WM, Lyons C, et al. Epidemiology of community-associated Clostridium difficile infection, 2009 through 2011. JAMA Intern Med. 2013;173(14):1359–67.
Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, Dumyati GK, Dunn JR, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(9):825–34.
Cohen NA, Miller T, Na'aminh W, Hod K, Adler A, Cohen D, et al. Clostridium difficile fecal toxin level is associated with disease severity and prognosis. United European Gastroenterol J. 2017;6(5):773–80.
Davies KA, Planche T, Wilcox MH. The predictive value of quantitative nucleic acid amplification detection of Clostridium difficile toxin gene for faecal sample toxin status and patient outcome. PLoS One. 2018;13(12):e0205941.
Garvey MI, et al. Can a toxin gene NAAT be used to predict toxin EIA and the severity of Clostridium difficile infection? Antimicrob Resist Infect Control. 2017;6(1):127.
Rao K, Santhosh K, Mogle JA, Higgins PD, Young VB. Elevated fecal calprotectin associates with adverse outcomes from Clostridium difficile infection in older adults. Infect Dis. 2016;48(9):663–9.
Davis MB, et al. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile–associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45(3):302–7.
Johnson S, et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, Controlled Trials. Clin Infect Dis. 2014;59(3):345–54.
Stevens VW, Nelson RE, Schwab-Daugherty EM, Khader K, Jones MM, Brown KA, et al. Comparative effectiveness of vancomycin and metronidazole for the prevention of recurrence and death in patients with Clostridium difficile infection. JAMA Intern Med. 2017;177(4):546–53.
Shah, S., B. Ereshefsky, L. Pontiggia, and M. Cawley. 2019. "Impact of Delayed Oral Vancomycin for Severe Clostridium difficile Infection." Hosp Pharm 54 (5):294-299. https://doi.org/10.1177/0018578718787439
Louie TJ, Miller MA, Mullane KM, Weiss K, Lentnek A, Golan Y, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364(5):422–31.
Cornely OA, Nathwani D, Ivanescu C, Odufowora-Sita O, Retsa P, Odeyemi IA. Clinical efficacy of fidaxomicin compared with vancomycin and metronidazole in Clostridium difficile infections: a meta-analysis and indirect treatment comparison. J Antimicrob Chemother. 2014;69(11):2892–900.
Fekety R, Silva J, Kauffman C, Buggy B, Deery HG. Treatment of antibiotic-associated Clostridium difficile colitis with oral vancomycin: comparison of two dosage regimens. Am J Med. 1989;86(1):15–9.
Lam SW, et al. Effect of vancomycin dose on treatment outcomes in severe Clostridium difficile infection. Int J Antimicrob Agents. 2013;42(6):553–8.
Rokas KE, Johnson JW, Beardsley JR, Ohl CA, Luther VP, Williamson JC. The addition of intravenous metronidazole to oral vancomycin is associated with improved mortality in critically ill patients with Clostridium difficile infection. Clin Infect Dis. 2015;61(6):934–41.
Malamood M, et al. Vancomycin enemas as adjunctive therapy for Clostridium difficile infection. J Clin Med Res. 2015;7(6):422–7.
Larson KC, Belliveau PP, Spooner LM. Tigecycline for the treatment of severe Clostridium difficile infection. Ann Pharmacother. 2011;45(7–8):1005–10.
Britt NS, et al. Tigecycline for the treatment of severe and severe complicated Clostridium difficile infection. Infect Dis Ther. 2014;3(2):321–31.
Coffman K, Chen XJ, Okamura C, Louie E. IVIG–A cure to severe refractory NAP-1 Clostridium difficile colitis? A case of successful treatment of severe infection, which failed standard therapy including fecal microbiota transplants and fidaxomicin. IDCases. 2017;8:27–8.
Bhangu A, Nepogodiev D, Gupta A, Torrance A, Singh P, West Midlands Research Collaborative. Systematic review and meta-analysis of outcomes following emergency surgery for Clostridium difficile colitis. Br J Surg. 2012;99(11):1501–13.
Steele SR, McCormick J, Melton GB, Paquette I, Rivadeneira DE, Stewart D, et al. Practice parameters for the management of Clostridium difficile infection. Dis Colon Rectum. 2015;58(1):10–24.
Lamontagne F, Labbé AC, Haeck O, Lesur O, Lalancette M, Patino C, et al. Impact of emergency colectomy on survival of patients with fulminant Clostridium difficile colitis during an epidemic caused by a hypervirulent strain. Ann Surg. 2007;245(2):267–72.
Bhangu S, Bhangu A, Nightingale P, Michael A. Mortality and risk stratification in patients with Clostridium difficile-associated diarrhoea. Color Dis. 2010;12(3):241–6.
Stewart DB, Hollenbeak CS, Wilson MZ. Is colectomy for fulminant Clostridium difficile colitis life saving? A systematic review. Color Dis. 2013;15(7):798–804.
Kulaylat AS, Kassam Z, Hollenbeak CS, Stewart DB Sr. A surgical Clostridium-associated risk of death score predicts mortality after colectomy for Clostridium difficile. Dis Colon Rectum. 2017;60(12):1285–90.
Neal MD, et al. Diverting loop ileostomy and colonic lavage: an alternative to total abdominal colectomy for the treatment of severe, complicated Clostridium difficile associated disease. Ann Surg. 2011;254(3):423–7 discussion 427-9.
Fashandi AZ, Martin AN, Wang PT, Hedrick TL, Friel CM, Smith PW, et al. An institutional comparison of total abdominal colectomy and diverting loop ileostomy and colonic lavage in the treatment of severe, complicated Clostridium difficile infections. Am J Surg. 2017;213(3):507–11.
Hall JF, Berger D. Outcome of colectomy for Clostridium difficile colitis: a plea for early surgical management. Am J Surg. 2008;196(3):384–8.
Hensgens MP, Dekkers OM, Goorhuis A, LeCessie S, Kuijper EJ. Predicting a complicated course of Clostridium difficile infection at the bedside. Clin Microbiol Infect. 2014;20(5):O301–8.
van der Wilden GM, et al. Fulminant Clostridium difficile colitis: prospective development of a risk scoring system. J Trauma Acute Care Surg. 2014;76(2):424–30.
Na X, Martin AJ, Sethi S, Kyne L, Garey KW, Flores SW, et al. A multi-center prospective derivation and validation of a clinical prediction tool for severe Clostridium difficile infection. PLoS One. 2015;10(4):e0123405.
Beauregard-Paultre C, et al. External validation of clinical scores to predict complications of Clostridium difficile infection. Open Forum Infect Dis. 2017;4(suppl_1):S402–2.
Kassam Z, Lee CH, Yuan Y, Hunt RH. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. Am J Gastroenterol. 2013;108(4):500–8.
Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis. 2011;53(10):994–1002.
Fischer M, Kao D, Kelly C, Kuchipudi A, Jafri SM, Blumenkehl M, et al. Fecal microbiota transplantation is safe and efficacious for recurrent or refractory Clostridium difficile infection in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2016;22(10):2402–9.
Kelly CR, Ihunnah C, Fischer M, Khoruts A, Surawicz C, Afzali A, et al. Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am J Gastroenterol. 2014;109(7):1065–71.
Cheng Y-W, et al. Mo1954 – Fecal microbiota transplantation is safe and effective for the treatment of Clostridium difficile infection in patients with liver cirrhosis. Gastroenterology. 2019;156(6):S-898–9.
Cheng, Y. W., E. Phelps, V. Ganapini, N. Khan, F. Ouyang, H. Xu, S. Khanna, R. Tariq, R. J. Friedman-Moraco, M. H. Woodworth, T. Dhere, C. S. Kraft, D. Kao, J. Smith, L. Le, N. El-Nachef, N. Kaur, S. Kowsika, A. Ehrlich, M. Smith, N. Safdar, E. A. Misch, J. R. Allegretti, A. Flynn, Z. Kassam, A. Sharfuddin, R. Vuppalanchi, and M. Fischer. 2019. "Fecal microbiota transplantation for the treatment of recurrent and severe Clostridium difficile infection in solid organ transplant recipients: A multicenter experience." Am J Transplant 19 (2):501-511. https://doi.org/10.1111/ajt.15058.
Chang JY, Antonopoulos DA, Kalra A, Tonelli A, Khalife WT, Schmidt TM, et al. Decreased diversity of the fecal microbiome in recurrent Clostridium difficile-associated diarrhea. J Infect Dis. 2008;197(3):435–8.
Wilson KH, Perini F. Role of competition for nutrients in suppression of Clostridium difficile by the colonic microflora. Infect Immun. 1988;56(10):2610–4.
Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157(1):121–41.
• Allegretti JR, et al. The 5D framework: a clinical primer for fecal microbiota transplantation to treat Clostridium difficile infection. Gastrointest Endosc. 2018;87(1):18–29. Clinically-focused discussion of patient/donor selection for FMT and infrastructure necessary for delivery.
Krajicek E, et al. Nuts and bolts of fecal microbiota transplantation. Clin Gastroenterol Hepatol. 2019;17(2):345–52.
Panchal P, Budree S, Scheeler A, Medina G, Seng M, Wong WF, et al. Scaling safe access to fecal microbiota transplantation: past, present, and future. Curr Gastroenterol Rep. 2018;20(4):14.
Agrawal M, Aroniadis OC, Brandt LJ, Kelly C, Freeman S, Surawicz C, et al. The long-term efficacy and safety of fecal microbiota transplant for recurrent, severe, and complicated Clostridium difficile infection in 146 elderly individuals. J Clin Gastroenterol. 2016;50(5):403–7.
Weingarden AR, et al. Resolution of severe Clostridium difficile infection following sequential fecal microbiota transplantation. J Clin Gastroenterol. 2013;47(8):735–7.
•• Fischer M, et al. Faecal microbiota transplantation plus selected use of vancomycin for severe-complicated Clostridium difficile infection: description of a protocol with high success rate. Aliment Pharmacol Ther. 2015;42(4):470–6. Describes a sequential FMT protocol with high rates of success in patients with SCDI and FCDI.
Cammarota G, et al. Randomised clinical trial: faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment Pharmacol Ther. 2015;41(9):835–43.
Fischer, M., B. Sipe, Y. W. Cheng, E. Phelps, N. Rogers, S. Sagi, M. Bohm, H. Xu, and Z. Kassam. 2017. "Fecal microbiota transplant in severe and severe-complicated Clostridium difficile: A promising treatment approach." Gut Microbes 8 (3):289-302. https://doi.org/10.1080/19490976.2016.1273998.
Ianiro G, Masucci L, Quaranta G, Simonelli C, Lopetuso LR, Sanguinetti M, et al. Randomised clinical trial: faecal microbiota transplantation by colonoscopy plus vancomycin for the treatment of severe refractory Clostridium difficile infection—single versus multiple infusions. Aliment Pharmacol Ther. 2018;48(2):152–9.
Cammarota G, Ianiro G, Magalini S, Gasbarrini A, Gui D. Decrease in surgery for Clostridium difficile infection after starting a program to transplant fecal microbiota. Ann Intern Med. 2015;163(6):487–8.
•• Hocquart M, et al. Early fecal microbiota transplantation improves survival in severe Clostridium difficile infections. Clin Infect Dis. 2018;66(5):645–50. Great discussion about use of FMT in SCDI despite lack of randomized control trials.
Cheng Y-W, et al. Inpatient fecal microbiota transplant program decreases mortality and colectomy in severe and/or complicated Clostridium difficile infection: 101. Am J Gastroenterol. 2017;112:S48–50.
Cheng Y-W, et al. Fecal microbiota transplant decreases mortality in patients with refractory severe and severe complicated Clostridium difficile infection Not Eligible for Colectomy: 2017 Fellows-in-Training Award (Colon Category): 100. Am J Gastroenterol. 2017;112:S48–50.
Napolitano, L. M., and C. E. Edmiston, Jr. 2017. "Clostridium difficile disease: Diagnosis, pathogenesis, and treatment update." Surgery 162 (2):325-348. https://doi.org/10.1016/j.surg.2017.01.018.
Pépin J, Valiquette L, Cossette B. Mortality attributable to nosocomial Clostridium difficile–associated disease during an epidemic caused by a hypervirulent strain in Quebec. Can Med Assoc J. 2005;173(9):1037–42.
DePestel DD, Aronoff DM. Epidemiology of Clostridium difficile infection. J Pharm Pract. 2013;26(5):464–75.
Franceschi C, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–54.
Lovat LB. Age related changes in gut physiology and nutritional status. Gut. 1996;38(3):306–9.
Minino AM, et al. Deaths: final data for 2008. Natl Vital Stat Rep. 2011;59(10):1–126.
Kasper AM, Nyazee HA, Yokoe DS, Mayer J, Mangino JE, Khan YM, et al. A multicenter study of Clostridium difficile infection-related colectomy, 2000-2006. Infect Control Hosp Epidemiol. 2012;33(5):470–6.
• Burke KE, Lamont JT. Fecal transplantation for recurrent Clostridium difficile infection in older adults: a review. J Am Geriatr Soc. 2013;61(8):1394–8. A systematic review of cure rates after FMT in elderly patients.
Li YT, Cai HF, Wang ZH, Xu J, Fang JY. Systematic review with meta-analysis: long-term outcomes of faecal microbiota transplantation for Clostridium difficile infection. Aliment Pharmacol Ther. 2016;43(4):445–57.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Yao-Wen Cheng declares that he has no conflict of interest. Monika Fischer declares that she has no conflict of interest.
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Gastroenterology for Geriatric Patients
Rights and permissions
About this article
Cite this article
Cheng, YW., Fischer, M. Treatment of Severe and Fulminnant Clostridioides difficile Infection. Curr Treat Options Gastro 17, 524–533 (2019). https://doi.org/10.1007/s11938-019-00262-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11938-019-00262-1