Abstract
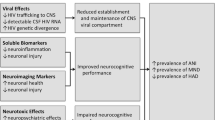
Effective combination antiretroviral therapy has transformed HIV infection into a chronic disease, with HIV-infected individuals living longer and reaching older age. Neurological disease remains common in treated HIV, however, due in part to ongoing inflammation and immune activation that persist in chronic infection. In this review, we highlight recent developments in our understanding of several clinically relevant neurologic complications that can occur in HIV infection despite treatment, including HIV-associated neurocognitive disorders, symptomatic CSF escape, cerebrovascular disease, and peripheral neuropathy.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Zaidi J, Grapsa E, Tanser F, Newell ML, Barnighausen T. Dramatic increase in HIV prevalence after scale-up of antiretroviral treatment. AIDS. 2013;27:2301–5.
Ortblad KF, Lozano R, Murray CJ. The burden of HIV: insights from the Global Burden of Disease Study 2010. AIDS. 2013;27:2003–17.
Spudich S, Gisslen M, Hagberg L, Lee E, Liegler T, Brew B, et al. Central nervous system immune activation characterizes primary human immunodeficiency virus 1 infection even in participants with minimal cerebrospinal fluid viral burden. J Infect Dis. 2011;204:753–60.
Valcour V, Chalermchai T, Sailasuta N, Marovich M, Lerdlum S, Suttichom D, et al. Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis. 2012;206:275–82.
Yilmaz A, Price RW, Spudich S, Fuchs D, Hagberg L, Gisslen M. Persistent intrathecal immune activation in HIV-1-infected individuals on antiretroviral therapy. J Acquir Immune Defic Syndr. 2008;47:168–73.
Price RW, Peterson J, Fuchs D, Angel TE, Zetterberg H, Hagberg L, et al. Approach to cerebrospinal fluid (CSF) biomarker discovery and evaluation in HIV infection. J Neuroimmune Pharmacol. 2013;8:1147–58. A detailed summary of the utility of CSF biomarkers as a window into the pathobiology, diagnosis and assessment of response to treatment in CNS involvement of HIV infection.
Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81.
Price RW. Neurological complications of HIV infection. Lancet. 1996;348:445–52.
Heaton RK, Clifford DB, Franklin Jr DR, Woods SP, Ake C, Vaida F, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–96.
Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J, et al. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS. 2007;21:1915–21.
Simioni S, Cavassini M, Annoni JM, Rimbault Abraham A, Bourquin I, Schiffer V, et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS. 2010;24:1243–50.
Meyer AC, Boscardin WJ, Kwasa JK, Price RW. Is it time to rethink how neuropsychological tests are used to diagnose mild forms of HIV-associated neurocognitive disorders? Impact of false-positive rates on prevalence and power. Neuroepidemiology. 2013;41:208–16. A thought-provoking perspective on the potential misclassifcation of milder forms of HIV-associated neurocognitive disorders using currently accepted definitions and the impact of high false positive frequencies on prevalence estimates.
Gorman AA, Foley JM, Ettenhofer ML, Hinkin CH, van Gorp WG. Functional consequences of HIV-associated neuropsychological impairment. Neuropsychol Rev. 2009;19:186–203.
Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H, et al. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc. 2004;10:317–31.
Tozzi V, Balestra P, Murri R, Galgani S, Bellagamba R, Narciso P, et al. Neurocognitive impairment influences quality of life in HIV-infected patients receiving HAART. Int J STD AIDS. 2004;15:254–9.
Hinkin CH, Castellon SA, Durvasula RS, Hardy DJ, Lam MN, Mason KI, et al. Medication adherence among HIV+ adults: effects of cognitive dysfunction and regimen complexity. Neurology. 2002;59:1944–50.
Birbeck GL, Kvalsund MP, Byers PA, Bradbury R, Mang’ombe C, Organek N, et al. Neuropsychiatric and socioeconomic status impact antiretroviral adherence and mortality in rural Zambia. Am J Trop Med Hyg. 2011;85:782–9.
Andrade AS, Deutsch R, Celano SA, Duarte NA, Marcotte TD, Umlauf A, et al. Relationships among neurocognitive status, medication adherence measured by pharmacy refill records, and virologic suppression in HIV-infected persons. J Acquir Immune Defic Syndr. 2013;62:282–92.
Vivithanaporn P, Heo G, Gamble J, Krentz HB, Hoke A, Gill MJ, et al. Neurologic disease burden in treated HIV/AIDS predicts survival: a population-based study. Neurology. 2010;75:1150–8.
Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16.
Peluso MJ, Spudich S. Treatment of HIV in the CNS: effects of antiretroviral therapy and the promise of non-antiretroviral therapeutics. Curr HIV/AIDS Rep. 2014;11(3):353–62.
Burdo TH, Lackner A, Williams KC. Monocyte/macrophages and their role in HIV neuropathogenesis. Immunol Rev. 2013;254:102–13. This review outlines evidence in support of the widely accepted hypotheis that monocyte/macrophage activation in the CNS is a primary mechanism underlying neurological deficits in treated HIV infection.
Ellis RJ, Badiee J, Vaida F, Letendre S, Heaton RK, Clifford D, et al. CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS. 2011;25:1747–51.
Hellmuth J, Milanini B, Valcour V. Interactions between ageing and NeuroAIDS. Curr Opin HIV AIDS. 2014;9:527–32.
Masters MC, Ances BM. Role of neuroimaging in HIV-associated neurocognitive disorders. Semin Neurol. 2014;34:089–102. This review highlights several of the key imaging techniques used to evaluate HIV-associated neurocognitive disorders.
Becker JT, Cuesta P, Fabrizio M, Sudre G, Vergis EN, Douaihy A, et al. Brain structural and functional recovery following initiation of combination antiretroviral therapy. J Neurovirol. 2012;18:423–7.
Harezlak J, Buchthal S, Taylor M, Schifitto G, Zhong J, Daar E, et al. Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. AIDS. 2011;25:625–33.
Mohamed MA, Barker PB, Skolasky RL, Selnes OA, Moxley RT, Pomper MG, et al. Brain metabolism and cognitive impairment in HIV infection: a 3-T magnetic resonance spectroscopy study. Magn Reson Imaging. 2010;28:1251–7.
Plessis SD, Vink M, Joska JA, Koutsilieri E, Stein DJ, Emsley R. HIV infection and the fronto-striatal system: a systematic review and meta-analysis of fMRI studies. AIDS. 2014;28:803–11. This meta-analysis of functional imaging studies evaluating the impact of HIV on the brain identified consistent differences in the left inferior frontal gyrus and caudate nucleus between HIV-infected participants and uninfected controls across studies.
Caniglia EC, Cain LE, Justice A, Tate J, Logan R, Sabin C, et al. Antiretroviral penetration into the CNS and incidence of AIDS-defining neurologic conditions. Neurology. 2014;83:134–41. This large multinational observational cohort study found an association between intiation of an ART regimen with a high CNS penetration effectiveness (CPE) score and risk of HIV dementia. While these unexpected results may have been due to various limitations of the study, including channeling bias, they also underscore the ongoing controversy regarding the impact of treatment with an ART regimen with higher CPE score on cognitive function.
Ma Q, Vaida F, Wong J, Sanders CA, Kao YT, Croteau D, et al. Long-term efavirenz use is associated with worse neurocognitive functioning in HIV-infected patients. J Neurovirol. 2016;22:170–8.
Robertson KR, Su Z, Margolis DM, Krambrink A, Havlir DV, Evans S, et al. Neurocognitive effects of treatment interruption in stable HIV-positive patients in an observational cohort. Neurology. 2010;74:1260–6.
Dufour CA, Marquine MJ, Fazeli PL, Henry BL, Ellis RJ, Grant I, et al. Physical exercise is associated with less neurocognitive impairment among HIV-infected adults. J Neurovirol. 2013;19:410–7.
Evering TH, Applebaum A, La Mar M, Garmon D, Dorfman D, Markowitz M. Rates of non-confounded HIV-associated neurocognitive disorders in men initiating combination antiretroviral therapy during primary infection. AIDS. 2016;30:203–10. Although limited by its small sample size, the exclusion of individuals with comorbid conditions that could confound or negatively impact cognitive function was a major strength of this cross-sectional study that demonstrated low rates of HIV-associated neurocognitive disorders in individuals who initiated ART during primary infection.
Crum-Cianflone NF, Moore DJ, Letendre S, Poehlman Roediger M, Eberly L, Weintrob A, et al. Low prevalence of neurocognitive impairment in early diagnosed and managed HIV-infected persons. Neurology. 2013;80:371–9.
Kore I, Ananworanich J, Valcour V, Fletcher JL, Chalermchai T, Paul R, et al. Neuropsychological impairment in acute HIV and the effect of immediate antiretroviral therapy. J Acquir Immune Defic Syndr. 2015;70:393–9.
Wright E, Grund B, Robertson K, Cysique L, Collins G, Brew B, et al. No difference between the effects of immediate versus deferred ART on neuropsychological test performance in HIV-positive adults with CD4+ cell counts > 500 cells/μL. Barcelona: Presented at the 15th European AIDS Conference; 2015.
Ndhlovu LC, Umaki T, Chew GM, Chow DC, Agsalda M, Kallianpur KJ, et al. Treatment intensification with maraviroc (CCR5 antagonist) leads to declines in CD16-expressing monocytes in cART-suppressed chronic HIV-infected subjects and is associated with improvements in neurocognitive test performance: implications for HIV-associated neurocognitive disease (HAND). J Neurovirol. 2014;20:571–82.
Gates TM, Cysique LA, Chaganti J, Siefried KJ, Brew BJ. Maraviroc-enhanced CART improves cognition in virally suppressed HAND: a pilot study. Seattle: Presented at the 22nd Conference on Retroviruses and Opportunistic Infections; 2015.
Letendre S, Marquie-Beck J, Capparelli E, Best B, Clifford D, Collier AC, et al. Validation of the CNS penetration-effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 2008;65:65–70.
Baker LM, Paul RH, Heaps-Woodruff JM, Chang JY, Ortega M, Margolin Z, et al. The effect of central nervous system penetration effectiveness of highly active antiretroviral therapy on neuropsychological performance and neuroimaging in HIV infected individuals. J Neuroimmune Pharmacol. 2015;10(3):487–92.
Fabbiani M, Grima P, Milanini B, Mondi A, Baldonero E, Ciccarelli N, et al. Antiretroviral neuropenetration scores better correlate with cognitive performance of HIV-infected patients after accounting for drug susceptibility. Antivir Ther. 2015;20:441–7.
Vassallo M, Durant J, Biscay V, Lebrun-Frenay C, Dunais B, Laffon M, et al. Can high central nervous system penetrating antiretroviral regimens protect against the onset of HIV-associated neurocognitive disorders? AIDS. 2014;28:493–501.
Marra CM, Zhao Y, Clifford DB, Letendre S, Evans S, Henry K, et al. Impact of combination antiretroviral therapy on cerebrospinal fluid HIV RNA and neurocognitive performance. AIDS. 2009;23:1359–66.
Libertone R, Lorenzini P, Balestra P, Pinnetti C, Ricottini M, Plazzi MM, et al. Central nervous system penetration-effectiveness rank does not reliably predict neurocognitive impairment in HIV-infected individuals. J Int AIDS Soc. 2014;17:19655.
Ellis RJ, Letendre S, Vaida F, Haubrich R, Heaton RK, Sacktor N, et al. Randomized trial of central nervous system-targeted antiretrovirals for HIV-associated neurocognitive disorder. Clin Infect Dis. 2014;58:1015–22. This small randomized controlled trial, which was terminated early, did not find a significant cognitive benefit of CNS-targeted compared with non-CNS-targeted ART regimens.
Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. Accessed 18 Mar 2016.
Canestri A, Lescure FX, Jaureguiberry S, Moulignier A, Amiel C, Marcelin AG, et al. Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin Infect Dis. 2010;50:773–8.
Peluso MJ, Ferretti F, Peterson J, Lee E, Fuchs D, Boschini A, et al. Cerebrospinal fluid HIV escape associated with progressive neurologic dysfunction in patients on antiretroviral therapy with well controlled plasma viral load. AIDS. 2012;26:1765–74.
Ferretti F, Gisslen M, Cinque P, Price RW. Cerebrospinal fluid HIV escape from antiretroviral therapy. Curr HIV/AIDS Rep. 2015;12:280–8. The authors review the poorly understood but well documented phenomenon of CSF escape in HIV.
Lescure FX, Moulignier A, Savatovsky J, Amiel C, Carcelain G, Molina JM, et al. CD8 encephalitis in HIV-infected patients receiving cART: a treatable entity. Clin Infect Dis. 2013;57:101–8.
Fois AF, Brew BJ. The potential of the CNS as a reservoir for HIV-1 infection: implications for HIV eradication. Curr HIV/AIDS Rep. 2015;12:299–303.
Chow FC, Regan S, Feske S, Meigs JB, Grinspoon SK, Triant VA. Comparison of ischemic stroke incidence in HIV-infected and non-HIV-infected patients in a US health care system. J Acquir Immune Defic Syndr. 2012;60:351–8.
Marcus JL, Leyden WA, Chao CR, Chow FC, Horberg MA, Hurley LB, et al. HIV infection and incidence of ischemic stroke. AIDS. 2014;28:1911–9.
Rasmussen LD, Engsig FN, Christensen H, Gerstoft J, Kronborg G, Pedersen C, et al. Risk of cerebrovascular events in persons with and without HIV. AIDS. 2011;25:1637–46.
Sico JJ, Chang CC, So-Armah K, Justice AC, Hylek E, Skanderson M, et al. HIV status and the risk of ischemic stroke among men. Neurology. 2015;84:1933–40. This study of the large, racially diverse Veterans Aging Cohort Study-Virtual Cohort confirmed findings from several other cohorts that HIV infection is an independent risk factor for ischemic stroke. Women, however, were excluded from the study.
Chow FC, He W, Bacchetti P, Regan S, Feske SK, Meigs JB, et al. Elevated rates of intracerebral hemorrhage in individuals from a US clinical care HIV cohort. Neurology. 2014;83:1705–11.
Durand M, Sheehy O, Baril J-G, LeLorier J, Tremblay CL. Risk of spontaneous intracranial hemorrhage in HIV-infected individuals: a population-based cohort study. J Stroke Cerebrovasc Dis. 2013;22:e34–41.
Hasse B, Ledergerber B, Furrer H, Battegay M, Hirschel B, Cavassini M, et al. Morbidity and aging in HIV-infected persons: the Swiss HIV Cohort Study. Clin Infect Dis. 2011;53:1130–9.
Schouten J, Wit FW, Stolte IG, Kootstra NA, van der Valk M, Geerlings SE, et al. Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: the AGEhIV cohort study. Clin Infect Dis. 2014;59:1787–97.
Womack JA, Chang CC, So-Armah KA, Alcorn C, Baker JV, Brown ST, et al. HIV infection and cardiovascular disease in women. J Am Heart Assoc. 2014;3, e001035.
Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–12.
Lang S, Mary-Krause M, Cotte L, Gilquin J, Partisani M, Simon A, et al. CO4 FHDoH-A: increased risk of myocardial infarction in HIV-infected patients in France, relative to the general population. AIDS. 2010;24:1228–30.
Chow FC, Wilson MR, Wu K, Ellis R, Bosch R, Linas B. Stroke incidence highest in women and black HIV-infected participants in ALLRT cohort. Boston: Presented at the 23nd Conference on Retroviruses and Opportunistic Infections; 2016.
Li JZ, Arnold KB, Lo J, Dugast AS, Plants J, Ribaudo HJ, et al. Differential levels of soluble inflammatory markers by human immunodeficiency virus controller status and demographics. Open Forum Infect Dis. 2015;2:117.
Martin GE, Gouillou M, Hearps AC, Angelovich TA, Cheng AC, Lynch F, et al. Age-associated changes in monocyte and innate immune activation markers occur more rapidly in HIV infected women. PLoS One. 2013;8, e55279.
Longenecker CT, Jiang Y, Orringer CE, Gilkeson RC, Debanne S, Funderburg NT, et al. Soluble CD14 is independently associated with coronary calcification and extent of subclinical vascular disease in treated HIV infection. AIDS. 2014;28:969–77.
Fitch KV, Srinivasa S, Abbara S, Burdo TH, Williams KC, Eneh P, et al. Noncalcified coronary atherosclerotic plaque and immune activation in HIV-infected women. J Infect Dis. 2013;208:1737–46. This study demonstrated differences in immune activation between HIV-infected women and men, with greater immune activation in HIV-infected women, which corresponded to a higher burden of noncalcified coronary plaque.
Westhorpe CLV, Maisa A, Spelman T, Hoy JF, Dewar EM, Karapanagiotidis S, et al. Associations between surface markers on blood monocytes and carotid atherosclerosis in HIV-positive individuals. Immunol Cell Biol. 2014;92:133–8.
McKibben RA, Margolick JB, Grinspoon S, Li X, Palella FJ, Kingsley LA, et al. Elevated levels of monocyte activation markers are associated with subclinical atherosclerosis in men with and those without HIV infection. J Infect Dis. 2015;211:1219–28.
Lo J, Lu MT, Ihenachor EJ, Wei J, Looby SE, Fitch KV, et al. Effects of statin therapy on coronary artery plaque volume and high-risk plaque morphology in HIV-infected patients with subclinical atherosclerosis: a randomised, double-blind, placebo-controlled trial. Lancet HIV. 2015;2:e52–63.
Curno MJ, Rossi S, Hodges-Mameletzis I, Johnston R, Price MA, Heidari S. A systematic review of the inclusion (or exclusion) of women in HIV research: from clinical studies of antiretrovirals and vaccines to cure strategies. J Acquir Immune Defic Syndr. 2016;71:181–8.
Gillis J, Smieja M, Cescon A, Rourke SB, Burchell AN, Cooper C, et al. Risk of cardiovascular disease associated with HCV and HBV coinfection among antiretroviral-treated HIV-infected individuals. Antivir Ther. 2014;19:309–17.
Freiberg MS, Cheng DM, Kraemer KL, Saitz R, Kuller LH, Samet JH. The association between hepatitis C infection and prevalent cardiovascular disease among HIV-infected individuals. AIDS. 2007;21:193–7.
Fernández-Montero JV, Barreiro P, de Mendoza C, Labarga P, Soriano V. Hepatitis C virus coinfection independently increases the risk of cardiovascular disease in HIV-positive patients. J Viral Hepat. 2016;23:47–52.
Bedimo R, Westfall AO, Mugavero M, Drechsler H, Khanna N, Saag M. Hepatitis C virus coinfection and the risk of cardiovascular disease among HIV-infected patients. HIV Med. 2010;11:462–8.
Sabin CA, Worm SW, Weber R, Reiss P, El-Sadr W, Dabis F, et al. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the D:A:D study: a multi-cohort collaboration. Lancet. 2008;371:1417–26.
Choi AI, Vittinghoff E, Deeks SG, Weekley CC, Li Y, Shlipak MG. Cardiovascular risks associated with abacavir and tenofovir exposure in HIV-infected persons. AIDS. 2011;25:1289–98.
Worm SW, Kamara DA, Reiss P, Fontas E, De Wit S, El-Sadr W, et al. Evaluation of HIV protease inhibitor use and the risk of sudden death or nonhemorrhagic stroke. J Infect Dis. 2012;205:535–9.
Gutierrez J, Goldman J, Dwork AJ, Elkind MS, Marshall RS, Morgello S. Brain arterial remodeling contribution to nonembolic brain infarcts in patients with HIV. Neurology. 2015;85:1139–45.
Soontornniyomkij V, Umlauf A, Chung SA, Cochran ML, Soontornniyomkij B, Gouaux B, et al. HIV protease inhibitor exposure predicts cerebral small vessel disease. AIDS. 2014;28:1297–306.
Chow FC, Bacchetti P, Kim AS, Price RW, Hsue PY. Effect of CD4+ cell count and viral suppression on risk of ischemic stroke in HIV infection. AIDS. 2014;28:2573–7.
Vinikoor MJ, Napravnik S, Floris-Moore M, Wilson S, Huang DY, Eron JJ. Incidence and clinical features of cerebrovascular disease among HIV-infected adults in the Southeastern United States. AIDS Res Hum Retroviruses. 2013;29:1068–74.
Sabin CA, Ryom L, De Wit S, Mocroft A, Phillips AN, Worm SW, et al. Associations between immune depression and cardiovascular events in HIV infection. AIDS. 2013;27:2735–48.
Duprez DA, Neuhaus J, Kuller LH, Tracy R, Belloso W, De Wit S, et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS One. 2012;7, e44454.
Tenorio AR, Zheng Y, Bosch RJ, Krishnan S, Rodriguez B, Hunt PW, et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis. 2014;210:1248–59. Several inflammatory markers predicted non-AIDS-defining events in this case–control study, including a composite of myocardial infarction and stroke.
Ghosh S, Chandran A, Jansen JP. Epidemiology of HIV-related neuropathy: a systematic literature review. AIDS Res Hum Retroviruses. 2012;28:36–48.
Robinson-Papp J, Sharma S, Simpson DM, Morgello S. Autonomic dysfunction is common in HIV and associated with distal symmetric polyneuropathy. J Neurovirol. 2013;19:172–80. This study of over 100 clinic-based HIV-infected patients investigated the prevalence of autonomic dysfunction in HIV and its association with distal symmetric neuropathy.
Kaku M, Simpson DM. HIV neuropathy. Curr Opin HIV AIDS. 2014;9:521–6. This is a comprehensive review of the clinical presenation and management of HIV associated neuropathy.
Geraci AP, Simpson DM. Neurological manifestations of HIV-1 infection in the HAART era. Compr Ther. 2001;27:232–41.
Lee AJ, Bosch RJ, Evans SR, Wu K, Harrison T, Grant P, et al. Patterns of peripheral neuropathy in ART-naive patients initiating modern ART regimen. J Neurovirol. 2015;21:210–8.
Simpson DM, Tagliati M. Nucleoside analogue-associated peripheral neuropathy in human immunodeficiency virus infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;9:153–61.
Brinkman K, ter Hofstede HJ, Burger DM, Smeitink JA, Koopmans PP. Adverse effects of reverse transcriptase inhibitors: mitochondrial toxicity as common pathway. AIDS. 1998;12:1735–44.
Lehmann HC, Chen W, Borzan J, Mankowski JL, Hoke A. Mitochondrial dysfunction in distal axons contributes to human immunodeficiency virus sensory neuropathy. Ann Neurol. 2011;69:100–10.
Pettersen JA, Jones G, Worthington C, Krentz HB, Keppler OT, Hoke A, et al. Sensory neuropathy in human immunodeficiency virus/acquired immunodeficiency syndrome patients: protease inhibitor-mediated neurotoxicity. Ann Neurol. 2006;59:816–24.
Ellis RJ, Marquie-Beck J, Delaney P, Alexander T, Clifford DB, McArthur JC, et al. Human immunodeficiency virus protease inhibitors and risk for peripheral neuropathy. Ann Neurol. 2008;64:566–72.
Chen H, Clifford DB, Deng L, Wu K, Lee AJ, Bosch RJ, et al. Peripheral neuropathy in ART-experienced patients: prevalence and risk factors. J Neurovirol. 2013;19:557–64.
Keswani SC, Polley M, Pardo CA, Griffin JW, McArthur JC, Hoke A. Schwann cell chemokine receptors mediate HIV-1 gp120 toxicity to sensory neurons. Ann Neurol. 2003;54:287–96.
Hao S. The molecular and pharmacological mechanisms of HIV-related neuropathic pain. Curr Neuropharmacol. 2013;11:499–512.
Wang SX, Ho EL, Grill M, Lee E, Peterson J, Robertson K, et al. Peripheral neuropathy in primary HIV infection associates with systemic and central nervous system immune activation. J Acquir Immune Defic Syndr. 2014;66:303–10. This study is one of the few that has focused on impairment of the peripheral nevous system in primary HIV infection. Peripheral neuropathy was prevalent in ART-naïve subjects and associated with blood and CSF markers of immune activation.
Pardo CA, McArthur JC, Griffin JW. HIV neuropathy: insights in the pathology of HIV peripheral nerve disease. J Peripher Nerv Syst. 2001;6:21–7.
Evans SR, Ellis RJ, Chen H, Yeh TM, Lee AJ, Schifitto G, et al. Peripheral neuropathy in HIV: prevalence and risk factors. AIDS. 2011;25:919–28.
Cornblath DR, Chaudhry V, Carter K, Lee D, Seysedadr M, Miernicki M, et al. Total neuropathy score: validation and reliability study. Neurology. 1999;53:1660–4.
Stavros K, Simpson DM. Understanding the etiology and management of HIV-associated peripheral neuropathy. Curr HIV/AIDS Rep. 2014;11:195–201.
Simpson DM, Brown S, Tobias JK, Vanhove GF. NGX-4010, a capsaicin 8 % dermal patch, for the treatment of painful HIV-associated distal sensory polyneuropathy: results of a 52-week open-label study. Clin J Pain. 2014;30:134–42.
Brown S, Simpson DM, Moyle G, Brew BJ, Schifitto G, Larbalestier N, et al. NGX-4010, a capsaicin 8% patch, for the treatment of painful HIV-associated distal sensory polyneuropathy: integrated analysis of two phase III, randomized, controlled trials. AIDS Res Ther. 2013;10:5. Evaluation of two phase III double-blinded clinical trials on the efficacy of a capsaicin 8% dermal patch for the treatment of HIV associated neuropathy found that a single 30 minute application of the patch provided significant pain relief for at least 12 weeks.
Abrams DI, Jay CA, Shade SB, Vizoso H, Reda H, Press S, et al. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology. 2007;68:515–21.
Ellis RJ, Toperoff W, Vaida F, van den Brande G, Gonzales J, Gouaux B, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology. 2009;34:672–80.
Youle M, Osio M. A double-blind, parallel-group, placebo-controlled, multicentre study of acetyl L-carnitine in the symptomatic treatment of antiretroviral toxic neuropathy in patients with HIV-1 infection. HIV Med. 2007;8:241–50.
McArthur JC, Yiannoutsos C, Simpson DM, Adornato BT, Singer EJ, Hollander H, et al. A phase II trial of nerve growth factor for sensory neuropathy associated with HIV infection. AIDS Clinical Trials Group Team 291. Neurology. 2000;54:1080–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Nisha S. Bhatia and Felicia C. Chow declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Infection
Rights and permissions
About this article
Cite this article
Bhatia, N.S., Chow, F.C. Neurologic Complications in Treated HIV-1 Infection. Curr Neurol Neurosci Rep 16, 62 (2016). https://doi.org/10.1007/s11910-016-0666-1
Published:
DOI: https://doi.org/10.1007/s11910-016-0666-1