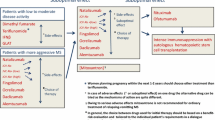
Abstract
Multiple sclerosis (MS), a demyelinating disease of the central nervous system, was untreatable until the mid-1990s when beta-interferons and glatiramer acetate were introduced. These agents, while effective, were relatively nonspecific in action. Over the last 10 years, research has focused toward developing more targeted therapies for the disease. Monoclonal antibodies (mAbs) have been central to these efforts and many of the mAbs studied in MS have been singularly effective. We review here the 6 monoclonal antibodies that have been approved for MS or are in late-stage clinical trials, focusing on the drugs’ efficacy and safety. Additionally, we review several monoclonal antibodies that were studied in MS but were found to be ineffective or even deleterious in this patient population.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–7.
Yednock TA, Cannon C, Fritz LC, Sanchez-Madrid F, Steinman L, Karin N. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature. 1992;356:63–6.
Kent SJ, Karlik SJ, Cannon C, Hines DK, Yednock TA, Fritz LC, et al. A monoclonal antibody to alpha 4 integrin suppresses and reverses active experimental allergic encephalomyelitis. J Neuroimmunol. 1995;58:1–10.
Kent SJ, Karlik SJ, Rice GP, Horner HC. A monoclonal antibody to alpha 4-integrin reverses the MR-detectable signs of experimental allergic encephalomyelitis in the guinea pig. J Magn Reson Imaging. 1995;5:535–40.
Sheremata WA, Vollmer TL, Stone LA, Willmer-Hulme AJ, Koller M. A safety and pharmacokinetic study of intravenous natalizumab in patients with MS. Neurology. 1999;52:1072–4.
Miller DH, Khan OA, Sheremata WA, Blumhardt LD, Rice GP, Libonati MA, et al. A controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2003;348:15–23.
Polman CH, O'Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910.
Rudick RA, Stuart WH, Calabresi PA, Confavreux C, Galetta SL, Radue EW, et al. Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med. 2006;354:911–23.
Langer-Gould A, Atlas SW, Green AJ, Bollen AW, Pelletier D. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med. 2005;353:375–81.
Kleinschmidt-DeMasters BK, Tyler KL. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N Engl J Med. 2005;353:369–74.
Biogen. www.medinfo.biogenidec.com [accessed May 6, 2013].
Gorelik L, Lerner M, Bixler S, Crossman M, Schlain B, Simon K, et al. Anti-JC virus antibodies: implications for PML risk stratification. Ann Neurol. 2010;68:295–303.
• Bloomgren G, Richman S, Hotermans C, Subramanyam M, Goelz S, Natarajan A, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med. 2012;366:1870–80. The authors stratify the risk for PML according to known risk factors of JC virus seropositivity, prior use of immunosuppressants, and duration of natalizumab therapy. They provide up-to-date estimates of PML risk in patients with any combination of these risk factors.
Sørensen PS, Bertolotto A, Edan G, Giovannoni G, Gold R, Havrdova E, et al. Risk stratification for progressive multifocal leukoencephalopathy in patients treated with natalizumab. Mult Scler. 2012;18:143–52.
Clifford DB, De Luca A, DeLuca A, Simpson DM, Arendt G, Giovannoni G, et al. Natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: lessons from 28 cases. Lancet Neurol. 2010;9:438–46.
Phan-Ba R, Lommers E, Tshibanda L, Calay P, Dubois B, Moonen G, et al. MRI preclinical detection and asymptomatic course of a progressive multifocal leucoencephalopathy (PML) under natalizumab therapy. J Neurol Neurosurg Psychiatry. 2012;83:224–6.
Blair NF, Brew BJ, Halpern JP. Natalizumab-associated PML identified in the presymptomatic phase using MRI surveillance. Neurology. 2012;78:507–8.
Ayzenberg I, Lukas C, Trampe N, Gold R, Hellwig K. Value of MRI as a surrogate marker for PML in natalizumab long-term therapy. J Neurol. 2012;259:1732–3.
Lindå H, von Heijne A. Presymptomatic diagnosis with MRI and adequate treatment ameliorate the outcome after natalizumab-associated progressive multifocal leukoencephalopathy. Front Neurol. 2013;4:11.
Khatri BO, Man S, Giovannoni G, Koo AP, Lee JC, Tucky B, et al. Effect of plasma exchange in accelerating natalizumab clearance and restoring leukocyte function. Neurology. 2009;72:402–9.
Rispens T, Vennegoor A, Wolbink GJ, Polman CH, Killestein J. Natalizumab remains detectable in patients with multiple sclerosis long after treatment is stopped. Mult Scler. 2012;18:899–901.
Miravalle A, Jensen R, Kinkel RP. Immune reconstitution inflammatory syndrome in patients with multiple sclerosis following cessation of natalizumab therapy. Arch Neurol. 2011;68:186–91.
Tan IL, McArthur JC, Clifford DB, Major EO, Nath A. Immune reconstitution inflammatory syndrome in natalizumab-associated PML. Neurology. 2011;77:1061–7.
Johnson T, Nath A. Immune reconstitution inflammatory syndrome and the central nervous system. Curr Opin Neurol. 2011;24:284–90.
Kleinschmidt-DeMasters BK, Miravalle A, Schowinsky J, Corboy J, Vollmer T. Update on PML and PML-IRIS occurring in multiple sclerosis patients treated with natalizumab. J Neuropathol Exp Neurol. 2012;71:604–17.
Rigau V, Mania A, Béfort P, Carlander B, Jonquet O, Lassmann H, et al. Lethal multiple sclerosis relapse after natalizumab withdrawal. Neurology. 2012;79:2214–6.
Kerbrat A, Le Page E, Leray E, Anani T, Coustans M, Desormeaux C, et al. Natalizumab and drug holiday in clinical practice: an observational study in very active relapsing remitting multiple sclerosis patients. J Neurol Sci. 2011;308:98–102.
Vellinga MM, Castelijns JA, Barkhof F, Uitdehaag BM, Polman CH. Postwithdrawal rebound increase in T2 lesional activity in natalizumab-treated MS patients. Neurology. 2008;70(13 Pt 2):1150–1.
Baumgartner A, Stich O, Rauer S. Clinical and radiological disease reactivation after cessation of long-term therapy with natalizumab. Int J Neurosci. 2012;122:35–9.
O'Connor PW, Goodman A, Kappos L, Lublin FD, Miller DH, Polman C, et al. Disease activity return during natalizumab treatment interruption in patients with multiple sclerosis. Neurology. 2011;76:1858–65.
Gilleece MH, Dexter TM. Effect of Campath-1H antibody on human hematopoietic progenitors in vitro. Blood. 1993;82:807–12.
Riechmann L, Clark M, Waldmann H, Winter G. Reshaping human antibodies for therapy. Nature. 1988;332:323–7.
Cox AL, Thompson SA, Jones JL, Robertson VH, Hale G, Waldmann H, et al. Lymphocyte homeostasis following therapeutic lymphocyte depletion in multiple sclerosis. Eur J Immunol. 2005;35:3332–42.
Thompson SA, Jones JL, Cox AL, Compston DA, Coles AJ. B-cell reconstitution and BAFF after alemtuzumab (Campath-1H) treatment of multiple sclerosis. J Clin Immunol. 2010;30:99–105.
Coles AJ, Cox A, Le Page E, Jones J, Trip SA, Deans J, et al. The window of therapeutic opportunity in multiple sclerosis: evidence from monoclonal antibody therapy. J Neurol. 2006;253:98–108.
Cossburn MD, Harding K, Ingram G, El-Shanawany T, Heaps A, Pickersgill TP, et al. Clinical relevance of differential lymphocyte recovery after alemtuzumab therapy for multiple sclerosis. Neurology. 2013;80:55–61.
Coles AJ, Fox E, Vladic A, Gazda SK, Brinar V, Selmaj KW, et al. Alemtuzumab more effective than interferon β-1a at 5-year follow-up of CAMMS223 clinical trial. Neurology. 2012;78:1069–78.
• Cohen JA, Coles AJ, Arnold DL, Confavreux C, Fox EJ, Hartung HP, et al. Alemtuzumab vs interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomized controlled phase 3 trial. Lancet. 2012;380:1819–28. In this phase 3 trial, the authors compare alemtuzumab to placebo in treatment naïve MS patients. They demonstrate fewer relapses, though no significant improvement in the accumulation of sustained disability, in patients taking alemtuzumab. The development of autoimmune diseases as a complication of alemtuzumab therapy underscores the need for close monitoring of patients on this drug.
• Coles AJ, Twyman CL, Arnold DL, Cohen JA, Confavreux C, Fox EJ, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomized controlled phase 3 trial. Lancet. 2012;380:1829–39. In this phase 3 trial, the authors compare alemtuzumab to standard therapy of IFNβ-1a. There were significantly fewer relapses and decreased accumulation of sustained disability in the alemtuzumab groups compared with IFNβ-1a, establishing the efficacy of alemtuzumab in patients who had a suboptimal response to existing first line therapies. Adverse events of autoimmune disease and increased infections were observed in the alemtuzumab group.
Coles AJ, Compston DA, Selmaj KW, Lake SL, Moran S, Margolin DH, et al. Alemtuzumab vs interferon beta-1a in early multiple sclerosis. N Engl J Med. 2008;359:1786–801.
Clatworthy MR, Wallin EF, Jayne DR. Anti-glomerular basement membrane disease after alemtuzumab. N Engl J Med. 2008;359:768–9.
Coles AJ. Alemtuzumab treatment of multiple sclerosis. Semin Neurol. 2013;33:66–73.
Somerfield J, Hill-Cawthorne GA, Lin A, Zandi MS, McCarthy C, Jones JL, et al. A novel strategy to reduce the immunogenicity of biological therapies. J Immunol. 2010;185:763–8.
Cossburn M, Pace AA, Jones J, Ali R, Ingram G, Baker K, et al. Autoimmune disease after alemtuzumab treatment for multiple sclerosis in a multicenter cohort. Neurology. 2011;77:573–9.
Malek TR, Castro I. Interleukin-2 receptor signaling: at the interface between tolerance and immunity. Immunity. 2010;33:153–65.
Wynn D, Kaufman M, Montalban X, Vollmer T, Simon J, Elkins J, et al. Daclizumab in active relapsing multiple sclerosis (CHOICE study): a phase 2, randomized, double-blind, placebo-controlled, add-on trial with interferon beta. Lancet Neurol. 2010;9:381–90.
• Gold R, Giovannoni G, Selmaj K, Havrdova E, Montalban X, Radue EW, et al. Daclizumab high-yield process in relapsing-remitting multiple sclerosis (SELECT): a randomized, double-blind, placebo-controlled trial. Lancet. 2013;381:2167–75. This phase 2 trial of daclizumab tested 2 doses of monthly subcutaneous daclizumab against placebo. It showed reduction in the annualized relapse rate and an increase in the number of patients who were relapse free for those on daclizumab compared with placebo. Notable adverse effects to the medication included infections (50%–54% in daclizumab-treated vs 44% in placebo) and cutaneous events (18%–22% in daclizumab-treated vs 13% in placebo). There was 1 death due to a psoas abscess in a patient on daclizumab.
US National Institute of Health. Efficacy and safety of daclizumab high yield process vs interferon beta-1a in patients with relapsing-remitting multiple sclerosis (DECIDE). Clinical trial identifier NCT01064401. www.clinicaltrials.gov. Accessed July 8, 2013.
Kabat E, Glusman M, Knaub V. Quantitative estimation of the albumin and gamma globulin in normal and pathologic cerebrospinal fluid by immunochemical methods. Am J Med. 1948;4:653–62.
Esiri MM. Multiple sclerosis: a quantitative and qualitative study of immunoglobulin-containing cells in the central nervous system. Neuropathol Appl Neurobiol. 1980;6:9–21.
Lucchinetti CF, Bruck W, Lassmann H. Evidence for pathogenic heterogeneity in multiple sclerosis. Ann Neurol. 2004;56:308.
Howell OW, Reeves CA, Nicholas R, Carassiti D, Radotra B, Gentleman SM, et al. Meningeal inflammation is widespread and linked to cortical pathology in multiple sclerosis. Brain. 2011;134(Pt 9):2755–71.
Stashenko P, Nadler LM, Hardy R, Schlossman SF. Characterization of a human B lymphocyte-specific antigen. J Immunol. 1980;125:1678–85.
Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358:676–88.
Naismith RT, Piccio L, Lyons JA, Lauber J, Tutlam NT, Parks BJ, et al. Rituximab add-on therapy for breakthrough relapsing multiple sclerosis: a 52-week phase II trial. Neurology. 2010;74:1860–7.
Hawker K, O'Connor P, Freedman MS, Calabresi PA, Antel J, Simon J, et al. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol. 2009;66:460–71.
Bar-Or A, Calabresi PA, Arnold D, Arnlod D, Markowitz C, Shafer S, et al. Rituximab in relapsing-remitting multiple sclerosis: a 72-week, open-label, phase I trial. Ann Neurol. 2008;63:395–400.
• Kappos L, Li D, Calabresi PA, O'Connor P, Bar-Or A, Barkhof F, et al. Ocrelizumab in relapsing-remitting multiple sclerosis: a phase 2, randomized, placebo-controlled, multicenter trial. Lancet. 2011;378:1779–87. This phase 2 trial compared 2 doses of ocrelizumab with placebo and weekly IFNβ-1a in patients with relapsing MS. By 8 weeks after study start, patients treated with either dose of ocrelizumab had dramatically (>80%) fewer gadolinium enhancing lesions on MRI than those treated with placebo or IFNβ-1a. No specific safety concerns were raised.
Roche. Press release, March 8, 2010.Available at: http://www.roche.com/media/media_releases/med-cor-2010-03-08.htm. Accessed June 12, 2013.
Teeling JL, Mackus WJ, Wiegman LJ, van den Brakel JH, Beers SA, French RR, et al. The biological activity of human CD20 monoclonal antibodies is linked to unique epitopes on CD20. J Immunol. 2006;177:362–71.
Rieckmann P, Albrecht M, Kitze B, Weber T, Tumani H, Broocks A, et al. Cytokine mRNA levels in mononuclear blood cells from patients with multiple sclerosis. Neurology. 1994;44:1523–6.
Rieckmann P, Albrecht M, Kitze B, Weber T, Tumani H, Broocks A, et al. Tumor necrosis factor-alpha messenger RNA expression in patients with relapsing-remitting multiple sclerosis is associated with disease activity. Ann Neurol. 1995;37:82–8.
Kuroda Y, Shimamoto Y. Human tumor necrosis factor-alpha augments experimental allergic encephalomyelitis in rats. J Neuroimmunol. 1991;34:159–64.
Ruddle NH, Bergman CM, McGrath KM, Lingenheld EG, Grunnet ML, Padula SJ, et al. An antibody to lymphotoxin and tumor necrosis factor prevents transfer of experimental allergic encephalomyelitis. J Exp Med. 1990;172:1193–200.
Selmaj K, Raine CS, Cross AH. Anti-tumor necrosis factor therapy abrogates autoimmune demyelination. Ann Neurol. 1991;30:694–700.
van Oosten BW, Barkhof F, Truyen L, Boringa JB, Bertelsmann FW, von Blomberg BM, et al. Increased MRI activity and immune activation in 2 multiple sclerosis patients treated with the monoclonal anti-tumor necrosis factor antibody cA2. Neurology. 1996;47:1531–4.
The Lenercept Multiple Sclerosis Study Group and The University of British Columbia MS/MRI Analysis Group. TNF neutralization in MS: results of a randomized, placebo-controlled multicenter study. Neurology. 1999;53:457–65.
Bosch X, Saiz A, Ramos-Casals M, Group BS. Monoclonal antibody therapy-associated neurological disorders. Nat Rev Neurol. 2011;7:165–72.
van Oosten BW, Lai M, Hodgkinson S, Barkhof F, Miller DH, Moseley IF, et al. Treatment of multiple sclerosis with the monoclonal anti-CD4 antibody cM-T412: results of a randomized, double-blind, placebo-controlled, MR-monitored phase II trial. Neurology. 1997;49:351–7.
Segal BM, Constantinescu CS, Raychaudhuri A, Kim L, Fidelus-Gort R, Kasper LH, et al. Repeated subcutaneous injections of IL12/23 p40 neutralising antibody, ustekinumab, in patients with relapsing-remitting multiple sclerosis: a phase II, double-blind, placebo-controlled, randomized, dose-ranging study. Lancet Neurol. 2008;7:796–804.
Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–8.
Segal BM, Dwyer BK, Shevach EM. An interleukin (IL)-10/IL-12 immunoregulatory circuit controls susceptibility to autoimmune disease. J Exp Med. 1998;187:537–46.
Brok HP, van Meurs M, Blezer E, Schantz A, Peritt D, Treacy G, et al. Prevention of experimental autoimmune encephalomyelitis in common marmosets using an anti-IL-12p40 monoclonal antibody. J Immunol. 2002;169:6554–63.
Acknowledgments
Erin E. Longbrake is supported by a Sylvia Lawry Fellowship from the National Multiple Sclerosis Society. Becky J. Parks has received grant support from the National MS Society, National Institutes of Health, and US Department of Defense. Anne H. Cross was supported in part by the Manny and Rosalyn Rosenthal-Dr. John Trotter Chair in Neuroimmunology of the Barnes-Jewish Hospital Foundation.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Erin E. Longbrake has received honoraria as an Alumni Presenter for 2013 Master MS Fellowship Program from Annenberg Center for Health Sciences; she has also received travel/accommodations expenses covered or reimbursed for 2013 Master MS Fellowship Program from the Annenberg Center for Health Sciences. Becky J. Parks serves on a volunteer position with the National MS Society Research Programs Advisory Committee; serves on a review committee for the Board of Scientific Counselors, National Institutes of Health; she has been a consultant for GlaxoSmithKline, Roche, QuestCor, Biogen Idec, Genzyme, Teva Neuroscience, Novartis. She has received honoraria from MS Society of Canada, Genzyme, CMEducation Resources, LLC, MedIQ; she has received payment for manuscript preparation from Massachusetts Medical Society and Journal of Internal Medicine. She has received payment for development of educational presentations including service on speakers' bureaus from Projects in Knowledge and CMEducation Resources, LLC; she has received payment for travel from herself only to and from consulting meetings for Biogen-Idec, Teva Neuroscience, Genzyme, Novartis, and Roche. Anne H. Cross has been a consultant for Biogen, Allergan, Questcor, Teva Neuroscience; has received grant support from Biogen Idec, Novartis, Allergan; has received honoraria from Biogen Idec, EMD Serono, Questcor, Teva Neuroscience, PRIME Education (CME organization), ProCE (CME organization); she has received payment for development of educational presentations including service on speakers' bureaus for PRIME. She has received travel expenses from Allergan, Biogen Idec, EMD Serono, Questcor, Teva Neuroscience. She owns stock that she bought on her own from Biogen Idec and Questcor and her children own stock in the same.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Demyelinating Disorders
Rights and permissions
About this article
Cite this article
Longbrake, E.E., Parks, B.J. & Cross, A.H. Monoclonal Antibodies as Disease Modifying Therapy in Multiple Sclerosis. Curr Neurol Neurosci Rep 13, 390 (2013). https://doi.org/10.1007/s11910-013-0390-z
Published:
DOI: https://doi.org/10.1007/s11910-013-0390-z