Abstract
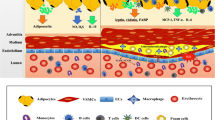
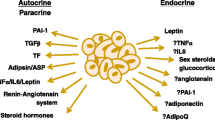
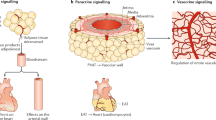
Perivascular adipose tissue (PVAT) is now recognized as an important paracrine organ influencing the homeostasis of the vessel wall, regional blood flow and peripheral arterial resistance. There is remarkable phenotypic variability and plasticity of PVAT among various vascular beds, exhibiting phenotypes from white to brown and beige adipocytes. PVAT dysfunction is characterized by disturbed secretion of various adipokines, which, together with endothelial dysfunction, contribute to hypertension and cardiovascular disease (CVD). This brief review describes our current knowledge on PVAT in health and cardiovascular disease, with a special focus on different phenotypes and signaling pathways in adipocytes of PVAT associated with hypertension, obesity and cardiovascular disorders.

Similar content being viewed by others
References
Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of Obesity Among Adults and Youth: United States, 2011-2014. NCHS Data Brief. 2015(219):1–8.
Gelber RP, Gaziano JM, Orav EJ, Manson JE, Buring JE, Kurth T. Measures of obesity and cardiovascular risk among men and women. J Am Coll Cardiol. 2008;52(8):605–15. doi:10.1016/j.jacc.2008.03.066.
Writing Group M, Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38–360. doi:10.1161/CIR.0000000000000350.
Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes. 2006;55(6):1537–45. doi:10.2337/db06-0263.
Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96(9):939–49. doi:10.1161/01.RES.0000163635.62927.34.
Lehman SJ, Massaro JM, Schlett CL, O'Donnell CJ, Hoffmann U, Fox CS. Peri-aortic fat, cardiovascular disease risk factors, and aortic calcification: the Framingham Heart Study. Atherosclerosis. 2010;210(2):656–61. doi:10.1016/j.atherosclerosis.2010.01.007.
Dupont J, Pollet-Villard X, Reverchon M, Mellouk N, Levy R. Adipokines in human reproduction. Horm Mol Biol Clin Investig. 2015;24(1):11–24. doi:10.1515/hmbci-2015-0034.
Abraham TM, Pedley A, Massaro JM, Hoffmann U, Fox CS. Association between visceral and subcutaneous adipose depots and incident cardiovascular disease risk factors. Circulation. 2015;132(17):1639–47. doi:10.1161/CIRCULATIONAHA.114.015000.
Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116(1):39–48. doi:10.1161/CIRCULATIONAHA.106.675355.
McLaughlin T, Lamendola C, Liu A, Abbasi F. Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. J Clin Endocrinol Metab. 2011;96(11):E1756–60. doi:10.1210/jc.2011-0615.
Katzmarzyk PT, Mire E, Bouchard C. Abdominal obesity and mortality: The Pennington Center Longitudinal Study. Nutr Diabetes. 2012;2, e42. doi:10.1038/nutd.2012.15.
Chatterjee TK, Stoll LL, Denning GM, Harrelson A, Blomkalns AL, Idelman G, et al. Proinflammatory phenotype of perivascular adipocytes: influence of high-fat feeding. Circ Res. 2009;104(4):541–9. doi:10.1161/CIRCRESAHA.108.182998.
Lee YC, Chang HH, Chiang CL, Liu CH, Yeh JI, Chen MF, et al. Role of perivascular adipose tissue-derived methyl palmitate in vascular tone regulation and pathogenesis of hypertension. Circulation. 2011;124(10):1160–71. doi:10.1161/CIRCULATIONAHA.111.027375.
Rittig K, Dolderer JH, Balletshofer B, Machann J, Schick F, Meile T, et al. The secretion pattern of perivascular fat cells is different from that of subcutaneous and visceral fat cells. Diabetologia. 2012;55(5):1514–25. doi:10.1007/s00125-012-2481-9.
Ozen G, Daci A, Norel X, Topal G. Human perivascular adipose tissue dysfunction as a cause of vascular disease: Focus on vascular tone and wall remodeling. Eur J Pharmacol. 2015;766:16–24. doi:10.1016/j.ejphar.2015.09.012.
Kagota S, Iwata S, Maruyama K, Wakuda H, Shinozuka K. Functional relationship between arterial tissue and perivascular adipose tissue in metabolic syndrome. Yakugaku Zasshi. 2016;136(5):693–7. doi:10.1248/yakushi.15-00262-2.
Nakamura K, Fuster JJ, Walsh K. Adipokines: a link between obesity and cardiovascular disease. J Cardiol. 2014;63(4):250–9. doi:10.1016/j.jjcc.2013.11.006.
Mahabadi AA, Reinsch N, Lehmann N, Altenbernd J, Kalsch H, Seibel RM, et al. Association of pericoronary fat volume with atherosclerotic plaque burden in the underlying coronary artery: a segment analysis. Atherosclerosis. 2010;211(1):195–9. doi:10.1016/j.atherosclerosis.2010.02.013.
Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11(2):85–97. doi:10.1038/nri2921.
Mohammed MM, Myers DS, Sofola OA, Hainsworth R, Drinkhill MJ. Vasodilator effects of leptin on canine isolated mesenteric arteries and veins. Clin Exp Pharmacol Physiol. 2007;34(8):771–4. doi:10.1111/j.1440-1681.2007.04648.x.
Szasz T, Webb RC. Perivascular adipose tissue: more than just structural support. Clin Sci (Lond). 2012;122(1):1–12. doi:10.1042/CS20110151.
Wang P, Xu TY, Guan YF, Su DF, Fan GR, Miao CY. Perivascular adipose tissue-derived visfatin is a vascular smooth muscle cell growth factor: role of nicotinamide mononucleotide. Cardiovasc Res. 2009;81(2):370–80. doi:10.1093/cvr/cvn288.
Shields KJ, Stolz D, Watkins SC, Ahearn JM. Complement proteins C3 and C4 bind to collagen and elastin in the vascular wall: a potential role in vascular stiffness and atherosclerosis. Clin Trans Sci. 2011;4(3):146–52. doi:10.1111/j.1752-8062.2011.00304.x.
Galvez B, de Castro J, Herold D, Dubrovska G, Arribas S, Gonzalez MC, et al. Perivascular adipose tissue and mesenteric vascular function in spontaneously hypertensive rats. Arterioscler Thromb Vasc Biol. 2006;26(6):1297–302. doi:10.1161/01.ATV.0000220381.40739.dd.
Knudson JD, Dincer UD, Zhang C, Swafford Jr AN, Koshida R, Picchi A, et al. Leptin receptors are expressed in coronary arteries, and hyperleptinemia causes significant coronary endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2005;289(1):H48–56. doi:10.1152/ajpheart.01159.2004.
Van de Voorde J, Boydens C, Pauwels B, Decaluwe K. Perivascular adipose tissue, inflammation and vascular dysfunction in obesity. Curr Vasc Pharmacol. 2014;12(3):403–11.
Ketonen J, Shi J, Martonen E, Mervaala E. Periadventitial adipose tissue promotes endothelial dysfunction via oxidative stress in diet-induced obese C57Bl/6 mice. Circ J. 2010;74(7):1479–87.
Payne GA, Borbouse L, Kumar S, Neeb Z, Alloosh M, Sturek M, et al. Epicardial perivascular adipose-derived leptin exacerbates coronary endothelial dysfunction in metabolic syndrome via a protein kinase C-beta pathway. Arterioscler Thromb Vasc Biol. 2010;30(9):1711–7. doi:10.1161/ATVBAHA.110.210070.
Galvez-Prieto B, Dubrovska G, Cano MV, Delgado M, Aranguez I, Gonzalez MC, et al. A reduction in the amount and anti-contractile effect of periadventitial mesenteric adipose tissue precedes hypertension development in spontaneously hypertensive rats. Hypertens Res. 2008;31(7):1415–23. doi:10.1291/hypres.31.1415.
Chang J, Li Y, Huang Y, Lam KS, Hoo RL, Wong WT, et al. Adiponectin prevents diabetic premature senescence of endothelial progenitor cells and promotes endothelial repair by suppressing the p38 MAP kinase/p16INK4A signaling pathway. Diabetes. 2010;59(11):2949–59. doi:10.2337/db10-0582.
Zhang P, Wang Y, Fan Y, Tang Z, Wang N. Overexpression of adiponectin receptors potentiates the antiinflammatory action of subeffective dose of globular adiponectin in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2009;29(1):67–74. doi:10.1161/ATVBAHA.108.178061.
Hulsmans M, Van Dooren E, Mathieu C, Holvoet P. Decrease of miR-146b-5p in monocytes during obesity is associated with loss of the anti-inflammatory but not insulin signaling action of adiponectin. PLoS One. 2012;7(2), e32794. doi:10.1371/journal.pone.0032794.
Withers SB, Bussey CE, Saxton SN, Melrose HM, Watkins AE, Heagerty AM. Mechanisms of adiponectin-associated perivascular function in vascular disease. Arterioscler Thromb Vasc Biol. 2014;34(8):1637–42. doi:10.1161/ATVBAHA.114.303031.
Peri-Okonny PA, Ayers C, Maalouf N, Das SR, de Lemos JA, Berry JD, et al. Adiponectin protects against incident hypertension independent of body fat distribution: observations from the Dallas Heart Study. Diabetes Metab Res Rev. 2016. doi:10.1002/dmrr.2840.
Xu A, Vanhoutte PM. Adiponectin and adipocyte fatty acid binding protein in the pathogenesis of cardiovascular disease. Am J Physiol Heart Circ Physiol. 2012;302(6):H1231–40. doi:10.1152/ajpheart.00765.2011.
Sarvottam K, Magan D, Yadav RK, Mehta N, Mahapatra SC. Adiponectin, interleukin-6, and cardiovascular disease risk factors are modified by a short-term yoga-based lifestyle intervention in overweight and obese men. J Altern Complement Med. 2013;19(5):397–402. doi:10.1089/acm.2012.0086.
Goldstein BJ, Scalia RG, Ma XL. Protective vascular and myocardial effects of adiponectin. Nat Clin Pract Cardiovasc Med. 2009;6(1):27–35. doi:10.1038/ncpcardio1398.
Morita Y, Maeda K, Kondo T, Ishii H, Matsudaira K, Okumura N, et al. Impact of adiponectin and leptin on long-term adverse events in Japanese patients with acute myocardial infarction. Results from the Nagoya Acute Myocardial Infarction Study (NAMIS). Circ J. 2013;77(11):2778–85.
Wu ZJ, Cheng YJ, Gu WJ, Aung LH. Adiponectin is associated with increased mortality in patients with already established cardiovascular disease: a systematic review and meta-analysis. Metabolism. 2014;63(9):1157–66. doi:10.1016/j.metabol.2014.05.001.
Cheng KK, Lam KS, Wang Y, Huang Y, Carling D, Wu D, et al. Adiponectin-induced endothelial nitric oxide synthase activation and nitric oxide production are mediated by APPL1 in endothelial cells. Diabetes. 2007;56(5):1387–94. doi:10.2337/db06-1580.
Margaritis M, Antonopoulos AS, Digby J, Lee R, Reilly S, Coutinho P, et al. Interactions between vascular wall and perivascular adipose tissue reveal novel roles for adiponectin in the regulation of endothelial nitric oxide synthase function in human vessels. Circulation. 2013;127(22):2209–21. doi:10.1161/CIRCULATIONAHA.112.001133.
Yu L, Tu Q, Han Q, Zhang L, Sui L, Zheng L, et al. Adiponectin regulates bone marrow mesenchymal stem cell niche through a unique signal transduction pathway: an approach for treating bone disease in diabetes. Stem Cells. 2015;33(1):240–52. doi:10.1002/stem.1844.
Rocha VZ, Folco EJ, Ozdemir C, Sheikine Y, Christen T, Sukhova GK, et al. CXCR3 controls T-cell accumulation in fat inflammation. Arterioscler Thromb Vasc Biol. 2014;34(7):1374–81. doi:10.1161/ATVBAHA.113.303133.
van Stijn CM, Kim J, Barish GD, Tietge UJ, Tangirala RK. Adiponectin expression protects against angiotensin II-mediated inflammation and accelerated atherosclerosis. PLoS One. 2014;9(1), e86404. doi:10.1371/journal.pone.0086404.
Guo J, Bian Y, Bai R, Li H, Fu M, Xiao C. Globular adiponectin attenuates myocardial ischemia/reperfusion injury by upregulating endoplasmic reticulum Ca(2)(+)-ATPase activity and inhibiting endoplasmic reticulum stress. J Cardiovasc Pharmacol. 2013;62(2):143–53. doi:10.1097/FJC.0b013e31829521af.
Cao T, Gao Z, Gu L, Chen M, Yang B, Cao K, et al. AdipoR1/APPL1 potentiates the protective effects of globular adiponectin on angiotensin II-induced cardiac hypertrophy and fibrosis in neonatal rat atrial myocytes and fibroblasts. PLoS One. 2014;9(8), e103793. doi:10.1371/journal.pone.0103793.
Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395(6704):763–70. doi:10.1038/27376.
Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269(5223):543–6.
Myers Jr MG, Leibel RL, Seeley RJ, Schwartz MW. Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol Metab. 2010;21(11):643–51. doi:10.1016/j.tem.2010.08.002.
Huby AC, Otvos Jr L. Belin de Chantemele EJ. Leptin induces hypertension and endothelial dysfunction via aldosterone-dependent mechanisms in obese female mice. Hypertension. 2016;67(5):1020–8. doi:10.1161/HYPERTENSIONAHA.115.06642.
Beltowski J. Leptin and atherosclerosis. Atherosclerosis. 2006;189(1):47–60. doi:10.1016/j.atherosclerosis.2006.03.003.
Martin SS, Qasim AN, Rader DJ, Reilly MP. C-reactive protein modifies the association of plasma leptin with coronary calcium in asymptomatic overweight individuals. Obesity (Silver Spring). 2012;20(4):856–61. doi:10.1038/oby.2011.164.
Bickel C, Schnabel RB, Zeller T, Lackner KJ, Rupprecht HJ, Blankenberg S et al. Predictors of leptin concentration and association with cardiovascular risk in patients with coronary artery disease: results from the AtheroGene study. Biomarkers. 2016:1–9. doi:10.3109/1354750X.2015.1130745.
Amrock SM, Weitzman M. Effect of increased leptin and C-reactive protein levels on mortality: results from the National Health and Nutrition Examination Survey. Atherosclerosis. 2014;236(1):1–6. doi:10.1016/j.atherosclerosis.2014.06.009.
Petrini S, Neri T, Lombardi S, Cordazzo C, Balia C, Scalise V, et al. Leptin induces the generation of procoagulant, tissue factor bearing microparticles by human peripheral blood mononuclear cells. Biochim Biophys Acta. 2016;1860(6):1354–61. doi:10.1016/j.bbagen.2016.03.029.
Zlokovic BV, Jovanovic S, Miao W, Samara S, Verma S, Farrell CL. Differential regulation of leptin transport by the choroid plexus and blood-brain barrier and high affinity transport systems for entry into hypothalamus and across the blood-cerebrospinal fluid barrier. Endocrinology. 2000;141(4):1434–41. doi:10.1210/endo.141.4.7435.
Benkhoff S, Loot AE, Pierson I, Sturza A, Kohlstedt K, Fleming I, et al. Leptin potentiates endothelium-dependent relaxation by inducing endothelial expression of neuronal NO synthase. Arterioscler Thromb Vasc Biol. 2012;32(7):1605–12. doi:10.1161/ATVBAHA.112.251140.
Matsumoto T, Noguchi E, Ishida K, Nakayama N, Kobayashi T, Kamata K. Cilostazol improves endothelial dysfunction by increasing endothelium-derived hyperpolarizing factor response in mesenteric arteries from Type 2 diabetic rats. Eur J Pharmacol. 2008;599(1-3):102–9. doi:10.1016/j.ejphar.2008.10.006.
Beltowski J, Wojcicka G, Jamroz-Wisniewska A. Role of nitric oxide and endothelium-derived hyperpolarizing factor (EDHF) in the regulation of blood pressure by leptin in lean and obese rats. Life Sci. 2006;79(1):63–71. doi:10.1016/j.lfs.2005.12.041.
Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, et al. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111(3):305–17.
Wang J, Wang H, Luo W, Guo C, Wang J, Chen YE, et al. Leptin-induced endothelial dysfunction is mediated by sympathetic nervous system activity. J Am Heart Assoc. 2013;2(5), e000299. doi:10.1161/JAHA.113.000299.
Dubrovska G, Verlohren S, Luft FC, Gollasch M. Mechanisms of ADRF release from rat aortic adventitial adipose tissue. Am J Physiol Heart Circ Physiol. 2004;286(3):H1107–13. doi:10.1152/ajpheart.00656.2003.
Gollasch M. Vasodilator signals from perivascular adipose tissue. Br J Pharmacol. 2012;165(3):633–42. doi:10.1111/j.1476-5381.2011.01430.x.
Greenstein AS, Khavandi K, Withers SB, Sonoyama K, Clancy O, Jeziorska M, et al. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation. 2009;119(12):1661–70. doi:10.1161/CIRCULATIONAHA.108.821181.
DeBoer MD. Obesity, systemic inflammation, and increased risk for cardiovascular disease and diabetes among adolescents: a need for screening tools to target interventions. Nutrition. 2013;29(2):379–86. doi:10.1016/j.nut.2012.07.003.
Noonan DM, De Lerma BA, Vannini N, Mortara L, Albini A. Inflammation, inflammatory cells and angiogenesis: decisions and indecisions. Cancer Metastasis Rev. 2008;27(1):31–40. doi:10.1007/s10555-007-9108-5.
Nguyen Dinh Cat A, Briones AM, Callera GE, Yogi A, He Y, Montezano AC, et al. Adipocyte-derived factors regulate vascular smooth muscle cells through mineralocorticoid and glucocorticoid receptors. Hypertension. 2011;58(3):479–88. doi:10.1161/HYPERTENSIONAHA.110.168872.
Galvez-Prieto B, Bolbrinker J, Stucchi P, de Las Heras AI, Merino B, Arribas S, et al. Comparative expression analysis of the renin-angiotensin system components between white and brown perivascular adipose tissue. J Endocrinol. 2008;197(1):55–64. doi:10.1677/JOE-07-0284.
Lee RM, Lu C, Su LY, Gao YJ. Endothelium-dependent relaxation factor released by perivascular adipose tissue. J Hypertens. 2009;27(4):782–90. doi:10.1097/HJH.0b013e328324ed86.
Gao YJ, Takemori K, Su LY, An WS, Lu C, Sharma AM, et al. Perivascular adipose tissue promotes vasoconstriction: the role of superoxide anion. Cardiovasc Res. 2006;71(2):363–73. doi:10.1016/j.cardiores.2006.03.013.
Tano JY, Schleifenbaum J, Gollasch M. Perivascular adipose tissue, potassium channels, and vascular dysfunction. Arterioscler Thromb Vasc Biol. 2014;34(9):1827–30. doi:10.1161/ATVBAHA.114.303032.
Zavaritskaya O, Zhuravleva N, Schleifenbaum J, Gloe T, Devermann L, Kluge R, et al. Role of KCNQ channels in skeletal muscle arteries and periadventitial vascular dysfunction. Hypertension. 2013;61(1):151–9. doi:10.1161/HYPERTENSIONAHA.112.197566.
Gollasch M, Dubrovska G. Paracrine role for periadventitial adipose tissue in the regulation of arterial tone. Trends Pharmacol Sci. 2004;25(12):647–53. doi:10.1016/j.tips.2004.10.005.
Hausman DB, DiGirolamo M, Bartness TJ, Hausman GJ, Martin RJ. The biology of white adipocyte proliferation. Obes Rev. 2001;2(4):239–54.
Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293(2):E444–52. doi:10.1152/ajpendo.00691.2006.
Fitzgibbons TP, Kogan S, Aouadi M, Hendricks GM, Straubhaar J, Czech MP. Similarity of mouse perivascular and brown adipose tissues and their resistance to diet-induced inflammation. Am J Physiol Heart Circ Physiol. 2011;301(4):H1425–37. doi:10.1152/ajpheart.00376.2011.
Chatterjee TK, Aronow BJ, Tong WS, Manka D, Tang Y, Bogdanov VY, et al. Human coronary artery perivascular adipocytes overexpress genes responsible for regulating vascular morphology, inflammation, and hemostasis. Physiol Genomics. 2013;45(16):697–709. doi:10.1152/physiolgenomics.00042.2013.
Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150(2):366–76. doi:10.1016/j.cell.2012.05.016.
Richard D, Monge-Roffarello B, Chechi K, Labbe SM, Turcotte EE. Control and physiological determinants of sympathetically mediated brown adipose tissue thermogenesis. Front Endocrinol (Lausanne). 2012;3:36. doi:10.3389/fendo.2012.00036.
Sacks HS, Fain JN, Holman B, Cheema P, Chary A, Parks F, et al. Uncoupling protein-1 and related messenger ribonucleic acids in human epicardial and other adipose tissues: epicardial fat functioning as brown fat. J Clin Endocrinol Metab. 2009;94(9):3611–5. doi:10.1210/jc.2009-0571.
Bartelt A, Heeren J. The holy grail of metabolic disease: brown adipose tissue. Curr Opin Lipidol. 2012;23(3):190–5. doi:10.1097/MOL.0b013e328352dcef.
Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360(15):1509–17. doi:10.1056/NEJMoa0810780.
Chang L, Villacorta L, Li R, Hamblin M, Xu W, Dou C, et al. Loss of perivascular adipose tissue on peroxisome proliferator-activated receptor-gamma deletion in smooth muscle cells impairs intravascular thermoregulation and enhances atherosclerosis. Circulation. 2012;126(9):1067–78. doi:10.1161/CIRCULATIONAHA.112.104489.
Barbatelli G, Murano I, Madsen L, Hao Q, Jimenez M, Kristiansen K, et al. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metab. 2010;298(6):E1244–53. doi:10.1152/ajpendo.00600.2009.
Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem. 2010;285(10):7153–64. doi:10.1074/jbc.M109.053942.
Tran KV, Gealekman O, Frontini A, Zingaretti MC, Morroni M, Giordano A, et al. The vascular endothelium of the adipose tissue gives rise to both white and brown fat cells. Cell Metab. 2012;15(2):222–9. doi:10.1016/j.cmet.2012.01.008.
Lee P, Linderman JD, Smith S, Brychta RJ, Wang J, Idelson C, et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 2014;19(2):302–9. doi:10.1016/j.cmet.2013.12.017.
Hu R, He ML, Hu H, Yuan BX, Zang WJ, Lau CP, et al. Characterization of calcium signaling pathways in human preadipocytes. J Cell Physiol. 2009;220(3):765–70. doi:10.1002/jcp.21823.
Kassmann M, Harteneck C, Zhu Z, Nurnberg B, Tepel M, Gollasch M. Transient receptor potential vanilloid 1 (TRPV1), TRPV4, and the kidney. Acta Physiol (Oxf). 2013;207(3):546–64. doi:10.1111/apha.12051.
Palazzo E, Rossi F, de Novellis V, Maione S. Endogenous modulators of TRP channels. Curr Top Med Chem. 2013;13(3):398–407.
Shapovalov G, Lehen'kyi V, Skryma R, Prevarskaya N. TRP channels in cell survival and cell death in normal and transformed cells. Cell Calcium. 2011;50(3):295–302. doi:10.1016/j.ceca.2011.05.006.
Chen J, Li L, Li Y, Liang X, Sun Q, Yu H, et al. Activation of TRPV1 channel by dietary capsaicin improves visceral fat remodeling through connexin43-mediated Ca2+ influx. Cardiovasc Diabetol. 2015;14:22. doi:10.1186/s12933-015-0183-6.
Baskaran P, Krishnan V, Ren J, Thyagarajan B. Capsaicin induces browning of white adipose tissue and counters obesity by activating TRPV1 channel-dependent mechanisms. Br J Pharmacol. 2016;173(15):2369–89. doi:10.1111/bph.13514.
Baboota RK, Singh DP, Sarma SM, Kaur J, Sandhir R, Boparai RK, et al. Capsaicin induces "brite" phenotype in differentiating 3T3-L1 preadipocytes. PLoS One. 2014;9(7), e103093. doi:10.1371/journal.pone.0103093.
Bratz IN, Dick GM, Tune JD, Edwards JM, Neeb ZP, Dincer UD, et al. Impaired capsaicin-induced relaxation of coronary arteries in a porcine model of the metabolic syndrome. Am J Physiol Heart Circ Physiol. 2008;294(6):H2489–96. doi:10.1152/ajpheart.01191.2007.
Kark T, Bagi Z, Lizanecz E, Pasztor ET, Erdei N, Czikora A, et al. Tissue-specific regulation of microvascular diameter: opposite functional roles of neuronal and smooth muscle located vanilloid receptor-1. Mol Pharmacol. 2008;73(5):1405–12. doi:10.1124/mol.107.043323.
Cavanaugh DJ, Chesler AT, Jackson AC, Sigal YM, Yamanaka H, Grant R, et al. Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J Neurosci. 2011;31(13):5067–77. doi:10.1523/JNEUROSCI.6451-10.2011.
Schleifenbaum J, Kassmann M, Szijarto IA, Hercule HC, Tano JY, Weinert S, et al. Stretch-activation of angiotensin II type 1a receptors contributes to the myogenic response of mouse mesenteric and renal arteries. Circ Res. 2014;115(2):263–72. doi:10.1161/CIRCRESAHA.115.302882.
Che H, Yue J, Tse HF, Li GR. Functional TRPV and TRPM channels in human preadipocytes. Pflugers Arch. 2014;466(5):947–59. doi:10.1007/s00424-013-1355-4.
Sun W, Uchida K, Takahashi N, Iwata Y, Wakabayashi S, Goto T, et al. Activation of TRPV2 negatively regulates the differentiation of mouse brown adipocytes. Pflugers Arch. 2016;468(9):1527–40. doi:10.1007/s00424-016-1846-1.
Lynch FM, Withers SB, Yao Z, Werner ME, Edwards G, Weston AH, et al. Perivascular adipose tissue-derived adiponectin activates BK(Ca) channels to induce anticontractile responses. Am J Physiol Heart Circ Physiol. 2013;304(6):H786–95. doi:10.1152/ajpheart.00697.2012.
Weston AH, Egner I, Dong Y, Porter EL, Heagerty AM, Edwards G. Stimulated release of a hyperpolarizing factor (ADHF) from mesenteric artery perivascular adipose tissue: involvement of myocyte BKCa channels and adiponectin. Br J Pharmacol. 2013;169(7):1500–9. doi:10.1111/bph.12157.
Fesus G, Dubrovska G, Gorzelniak K, Kluge R, Huang Y, Luft FC, et al. Adiponectin is a novel humoral vasodilator. Cardiovasc Res. 2007;75(4):719–27. doi:10.1016/j.cardiores.2007.05.025.
Iwabu M, Yamauchi T, Okada-Iwabu M, Sato K, Nakagawa T, Funata M, et al. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature. 2010;464(7293):1313–9. doi:10.1038/nature08991.
Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, et al. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2(1):21–33. doi:10.1016/j.cmet.2005.06.005.
Perez GJ, Bonev AD, Nelson MT. Micromolar Ca(2+) from sparks activates Ca(2+)-sensitive K(+) channels in rat cerebral artery smooth muscle. Am J Physiol Cell Physiol. 2001;281(6):C1769–75.
Lohn M, Dubrovska G, Lauterbach B, Luft FC, Gollasch M, Sharma AM. Periadventitial fat releases a vascular relaxing factor. FASEB J. 2002;16(9):1057–63. doi:10.1096/fj.02-0024com.
Schleifenbaum J, Kohn C, Voblova N, Dubrovska G, Zavarirskaya O, Gloe T, et al. Systemic peripheral artery relaxation by KCNQ channel openers and hydrogen sulfide. J Hypertens. 2010;28(9):1875–82. doi:10.1097/HJH.0b013e32833c20d5.
Tsvetkov D, Tano JY, Kassmann M, Wang N, Schubert R, Gollasch M. The role of DPO-1 and XE991-sensitive potassium channels in perivascular adipose tissue-mediated regulation of vascular tone. Front Physiol. 2016;7:335. doi:10.3389/fphys.2016.00335.
Cheng Y, Ndisang JF, Tang G, Cao K, Wang R. Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am J Physiol Heart Circ Physiol. 2004;287(5):H2316–23. doi:10.1152/ajpheart.00331.2004.
Tang G, Wu L, Wang R. Interaction of hydrogen sulfide with ion channels. Clin Exp Pharmacol Physiol. 2010;37(7):753–63. doi:10.1111/j.1440-1681.2010.05351.x.
Kohn C, Schleifenbaum J, Szijarto IA, Marko L, Dubrovska G, Huang Y, et al. Differential effects of cystathionine-gamma-lyase-dependent vasodilatory H2S in periadventitial vasoregulation of rat and mouse aortas. PLoS One. 2012;7(8), e41951. doi:10.1371/journal.pone.0041951.
Verlohren S, Dubrovska G, Tsang SY, Essin K, Luft FC, Huang Y, et al. Visceral periadventitial adipose tissue regulates arterial tone of mesenteric arteries. Hypertension. 2004;44(3):271–6. doi:10.1161/01.HYP.0000140058.28994.ec.
Gollasch M. KCNQ channels and novel insights into coronary perfusion. Hypertension. 2013;62(6):1011–2. doi:10.1161/HYPERTENSIONAHA.113.01869.
Acknowledgments
The Deutsche Forschungsgemeinschaft (DFG) (MG), Deutsche Akademische Austauschdienst (DAAD) (MG) and Shanghai Tongji Hospital (XL) are supporting our studies. We thank Mario Kassmann and Dmitry Tsvetkov for critical reading and helpful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Drs. Lian and Gollasch declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Hypertension and Obesity
Rights and permissions
About this article
Cite this article
Lian, X., Gollasch, M. A Clinical Perspective: Contribution of Dysfunctional Perivascular Adipose Tissue (PVAT) to Cardiovascular Risk. Curr Hypertens Rep 18, 82 (2016). https://doi.org/10.1007/s11906-016-0692-z
Published:
DOI: https://doi.org/10.1007/s11906-016-0692-z