Abstract
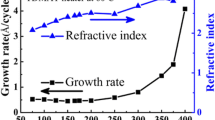
Ozone (O3) was employed as an oxygen source for the atomic layer deposition (ALD) of titanium dioxide (TiO2) based on tetrakis-dimethyl-amido titanium (TDMAT). The effects of deposition temperature and O3 feeding time on the film growth kinetics and physical/chemical properties of the TiO2 films were investigated. Film growth was possible at as low as 75 °C, and the growth rate (thickness/cycles) of TiO2 was minimally affected by varying the temperatures at 150–225 °C. Moreover, saturated growth behavior on the O3 feeding time was observed at longer than 0.5 s. Higher temperatures tend to provide films with lower levels of carbon impurities. The film thickness increased linearly as the number of cycles increased. With thicker films and at higher deposition temperatures, surface roughening tended to increase. The as-deposited films were amorphous regardless of the substrate temperatures and there was no change of crystal phase even after annealing at temperatures of 400–600 °C. The films deposited in 0.5 mm holes with an aspect ratio of 3: 1 showed an excellent conformality.
Similar content being viewed by others
References
H. A. Durand, J. H. Brimaud, O. Hellman, H. Shibata, S. Sakuragi, Y. Makita, D. Gesbert and P. Meyrueis, Appl. Surf. Sci., 86, 122, (1995).
J.-A. Jung, D. H. Kwak, D.W. Oh, D.M. Park and O.-B. Yang, Korean Chem. Eng. Res., 50, 11 (2012).
M. Keshmiri and T. Troczynski, J. Non-Crystal. Solids, 324, 289 (2003).
M.G. Choi, K.Y. Kang, Y.-G. Lee and K.M. Kim, Korean Chem. Eng. Res., 50, 25 (2012).
H. Kim, G. P. Kushto, C.B. Arnold, Z. H. Kafafi and A. Pique, Appl. Phys. Lett., 85, 64 (2004).
J. Yu, X. Zhao and Q. Zhao, Thin Solid Films, 379, 7 (2000).
M. O. Abou-Helal and W. T. Seeber, Appl. Surf. Sci., 195, 53 (2002).
M. Keshmiri, M. Mohseni and T. Troczynski, Appl. Catal., B53, 209 (2004).
H. Tada and M. Tanaka, Langmuir, 13, 360 (1997).
Y. Suda, H. Kawasaki, T. Ueda and T. Ohshima, Thin Solid Films, 453-54, 162 (2004).
H. Shin, D.-K. Jeong, J. Lee, M.M. Sung and J. Kim, Adv. Mater., 16, 1197 (2004).
J.W. Lim, S. J. Yun and J. H. Lee, Electrochem. and Solid-State Lett., 7, F73 (2004).
J. Dendooven, S. P. Sree, K. D. Keyser, D. Deduytsche, J. A. Martens, K. F. Ludwig and C. Detavernier, J. Phys. Chem. C, 115, 6605 (2011).
S. K. Kim, S. Hoffmann-Eifert, M. Reiners and R. Waser, J. Electrochem. Soc., 158, D6 (2011).
Q. Xie, J. Musschoot, D. Deduytsche, R. L. Van Meirhaeghe, C. Detavernier, S.V. Berghe, Y.-L. Jiang, G.-P. Ru, B.-Z. Li and X.-P. Qu, J. Electrochem. Soc., 155, H688 (2008).
V. R. Rai and S. Agarwal, J. Phys. Chem. C, 112, 9552 (2008).
S. K. Kim, S.Y. Lee, M. Seo, G.-J. Choi and C. S. Hwang, J. Appl. Phys., 102, 024109 (2007).
R. Pheamhom, C. Sunwoo and D. H. Kim, J. Vac. Sci. Technol. A, 24, 1535 (2006).
G. T. Lim and D. H. Kim, Thin Soild Films, 498, 254 (2006).
M. Rose, J. Niinisto, P. Michalowski, L. Gerlich, L. Wilde, I. Endler and J.W. Bartha, J. Phys. Chem. C, 113, 21825 (2009).
X. Liu, S. Ramanathan, A. Longdergan, A. Srivastava, D. Lee, T. E. Seidel, J. F. Barton, D. Pang and R.G. Gordon, J. Electrochem. Soc., 152, G213 (2005).
R. L. Puurunen, J. Appl. Phys., 97, 121301 (2005).
S. E. Potts, W. Keuning, E. Langereis, G. Dingemans, M.C. M. van de Sanden and W.M. M. Kessels, J. Electrochem. Sco., 157, 66 (2010).
Y. Wang, M. Dai, M.-T. Ho, L. S. Wielunski and Y. J. Chabal, Appl. Phys. Lett., 90, 22906 (2007).
G. R. Torres, T. Lindgren, J. Lu, C.G. Granqvist and S. E. Lindquist, J. Phys. Chem. B, 108, 5995 (2004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, YW., Kim, DH. Atomic layer deposition of TiO2 from tetrakis-dimethylamido-titanium and ozone. Korean J. Chem. Eng. 29, 969–973 (2012). https://doi.org/10.1007/s11814-012-0072-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-012-0072-6