Abstract
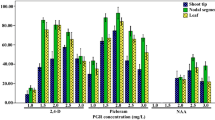
An in vitro plant regeneration system was established from the spores of Pteris vittata and identification of its tolerance, and accumulation of gametophytes and callous, to arsenic (As) and copper (Cu) was investigated. The highest frequency (100%) of callus formation was achieved from gametophyte explants treated with 0.5 mg l−1 6-benzylaminopurine (6-BA) + 0.5 mg l−1 gibberellin acid (GA). Furthermore, sporophytes were differentiated from the callus tissue derived from gametophyte explants on MS medium supplemented with 0.5 mg l−1 6-BA, 0.5–1.0 mg l−1 GA and additional 300 mg l−1 lactalbumin hydrolysate (LH) for 4 weeks. The optimum combination of ½ MS + 1.0 mg l−1 GA + 0.5 mg l−1 6-BA + 300 mg l−1 LH promoted sporophyte formation on 75 ± 10% of the callus. Every callus derived from gametophyte explants could achieve 3–4 sporophytes. The in vitro growth of gametophyte and callus was accelerated in the medium containing Na3AsO4 lower than 0.5 mM, but this growth was inhibited with 2 mM Na3AsO4. And with the increase of Na3AsO4 in the culture medium from 0 to 2 mM, the As accumulation in gametophytes and callus increased and achieved a level of 763.3 and 315.4 mg kg−1, respectively. Gametophytes and calluses transplanted to culture medium, supplemented with different concentrations of CuSO4, are similar to those in Na3AsO4, and the Cu accumulation in gametophytes could achieve 7,940 mg kg−1 when gametophytes were subcultured in medium containing 3 mM CuSO4. These results suggested that the high efficiency propagation system could be a useful and rapid means to identify other heavy metal tolerance and accumulation. Further, the regeneration ability of callus made it possible for genetic transformation of this fern.




Similar content being viewed by others
Abbreviations
- MS:
-
Murashige and Skoog 1962
- 6-BA:
-
6-Benzylaminopurine
- GA:
-
Gibberellin acid
- LH:
-
Lactalbumin hydrolysate
- 2, 4-D:
-
2, 4-dichlorophenoxy acetic acid
References
Bhaskar R, Bondada ST, Ma LQ (2004) Absorption of foliar-applied arsenic by the arsenic hyperaccumulating fern (Pteris vittata L.). Sci Total Environ 332:61–70
Breznovits A, Mohay J (1987) In vitro problems related to propagation of different fern species. Acta Hortic 212:427–431
Bueno P, Piqueras A (2002) Effect of transition metals on stress, lipid peroxidation and antioxidant enzyme activities in tobacco cell culture. Plant Growth Regul 36:161–167
Caille N, Zhao FJ, McGrath SP (2005) Comparison of root absorption, translocation and tolerance of arsenic in the hyperaccumulator Pteris vittata and the nonhyperaccumulator Pteris tremula. New Phytol 165:755–761
Fay MF (1994) In what situations is in vitro culture appropriate to plant conservation? Biodivers Conserv 3:176–183
Fernández H, Bertrand AM, Sánchez-Tamés R (1997a) Gemmation in Osmunda regalis L. gametophyte cultured in vitro. Plant Cell Rep 16:358–362
Fernández H, Bertrand AM, Sánchez-Tamés R (1997b) Plantlet regeneration in Asplenium nidus L. and Pteris ensiformis L. by homogenisation of BA treated rhizomes. Sci Hortic 68:243–247
Fernández H, Bertrand AM, Sierra MI, Sánchez-Tamés R (1999) An apolar GA-like compound responsible for the antheridiogen activity in Blechnum spicant. Plant Growth Regul 28:143–144
Hassanein A, Dorion N (2005) Efficient plant regeneration system from leaf discs of zonal (Pelargonium xhortorum) and two scented (P. capitatum and P. graveolens) geraniums. Plant Cell Tissue Organ Cult 83:231–240
Kurepa J, Van Montagu M, Inzé D (1997) Expression of sodCp and sodB genes in Nicotians tabacum: effects of light and copper excess. J Exp Bot 48:2007–2014
Ma LQ, Komar KMM, Tu C, Zhang W, Cai Y, Kennelley ED (2001) A fern that hyperaccumulates arsenic. Nature 409:579
Meharg AA (2004) Arsenic in rice: understanding a new disaster for South-East Asia. Trends Plant Sci 9:415–417
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Plant Physiol 15:473–497
Pierik RML, Oerlemans APC, Verhoeven MAP, Mentjox MA, Molenberg H (1986) Zaai van varens in kweekbuizen. Vakbl Bloemisterij 10:48–49
Salido AL, Hasty KL, Lim JM, Butcher DJ (2003) Phytoremediation of arsenic and lead in contaminated soil using Chinese brake ferns (Pteris vittata) and Indian mustard (Brassica juncea). Int J Phytoremediation 5:89–103
Salt DE, Krämer U (1999) Mechanisms of metal hyperaccumulation in plants. In: Ensley BD, Raskin I (eds) Phytoremediation of toxic metals: using plants to clean up the environment. Wiley, New York, pp 231–246
Sheffield E, Bell PR (1987) Current studies of the pteridophyte life cycle. Bot Rev 53:442–490
Smith AH, Hopenhayn RC, Bates MN, Goeden HM, Hertz PI, Duggan HM, Wood R, Kosnett MJ, Smith MT (1992) Cancer risks from arsenic in drinking water. Environ Health Perspect 97:259–267
Swami P, Raghavan V (1980) Control of morphogenesis in the gametophyte of a fern by light and growth hormones. Can J Bot 58:1464–1473
Acknowledgments
This work was supported by the National Natural Science Foundation of China (30570138, 30500307).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Tukiendorf.
Rights and permissions
About this article
Cite this article
Zheng, Y., Xu, W., He, Z. et al. Plant regeneration of the arsenic hyperaccumulator Pteris vittata L. from spores and identification of its tolerance and accumulation of arsenic and copper. Acta Physiol Plant 30, 249–255 (2008). https://doi.org/10.1007/s11738-007-0114-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-007-0114-6