Abstract
Unconjugated ynone derivatives with the 1,3-dicarbonyl structure are synthesized, and they are directly subjected to gold-catalyzed reaction with hydroxylamine without obtaining the oxime derivative. Among the different metal salts investigated, AuCl3 is the best for yielding and obtaining two different heterocyclic skeletons. Thanks to this reaction, two important heterocyclic molecules, 2H-1,2-oxazine and 1-hydroxypyrrole, are obtained as a one-pot and single-step strategy with favorable yields. Eleven different 2H-1,2-oxazine and 6 different 1-hydroxypyrrole derivatives are synthesized using starting compounds containing different substituents, in which steric hindrance and electronic effect are also taken into account. When the same reaction is carried out with the unconjugated ynone derivative containing only one carbonyl group, the only 1-hydroxypyrrole derivative is observed to be formed, and 1,2-oxazine ring is not observed.
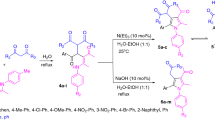
Graphical abstract







Similar content being viewed by others
References
Acheson RM (1990) 1-hydroxypyrroles, 1-hydroxyindoles and 9 hydroxycarbazoles. Adv Heterocycl Chem 51:105–175. https://doi.org/10.1016/S0065-2725(08)60002-1
Alcaide B, Almendros P, Alonso JM (2011) Gold-Catalyzed cyclizations of alkynol-based compounds: synthesis of natural products and derivatives. Molecules 16:7815–7843. https://doi.org/10.3390/molecules16097815
Ashton JAR, Clarke AK, Taylor RJK, Unsworth WP (2020) Modular synthesis of polycyclic alkaloid scaffolds via an enantioselective dearomative cascade. Org Lett 22(3):1175–1181. https://doi.org/10.1021/acs.orglett.0c00053
Bhatt D, Chae S, Kim HY, Oh K (2022) One-pot synthesis of n-hydroxypyrroles via soft α-vinyl enolization of (E)-β-chlorovinyl ketones: a traceless arylsulfinate mediator strategy. Org Lett 24:2636–2640. https://doi.org/10.1021/acs.orglett.2c00649
Brandstatter M, Huwyler N, Carreira EM (2019) Gold(I)-catalyzed stereoselective cyclization of 1,3-enyne aldehydes by a 1,3-acyloxy migration/Nazarov cyclization/aldol addition cascade. Chem Sci 10:8219–8223. https://doi.org/10.1039/C9SC02828E
Chada RR, Kajare RC, Bhandari MC, Mohammed SZ, Khatravath M, Warudikar K, Punna N (2021) Facile access to [1,2]-oxazine derivatives via annulations of aminoxy-tethered 1,7-enynes. Org Bio Chem 19:809–821. https://doi.org/10.1039/D0OB02279A
Chen Y, Zhang D, Sun M, Ding Z (2022) Divergent synthesis of isoxazoles and 6H–1,2-oxazines via hypervalent iodine-mediated intramolecular oxygenation of alkenes. New J Chem 46:5663–5667. https://doi.org/10.1039/D1NJ05922J
Cordier M, Archambeau A (2018) (3 + 3) cycloaddition of oxyallyl cations with nitrones: diastereoselective access to 1,2-oxazinanes. Org Lett 20(8):2265–2268. https://doi.org/10.1021/acs.orglett.8b00617
Cowper NGW, Hesse MJ, Chan KM, Reisman S (2020) E (2020) A copper-catalyzed asymmetric oxime propargylation enables the synthesis of the gliovirin tetrahydro-1,2-oxazine core. Chem Sci 11:11897–11901. https://doi.org/10.1039/D0SC04802J
Dobrynin SA, Glazachev YI, Gatilov YV, Chernyak EI, Salnikov GE, Kirilyuk IA (2018) Synthesis of 3,4-Bis(hydroxymethyl)-2,2,5,5-tetraethylpyrrolidin-1-oxyl via 1,3-dipolar cycloaddition of azomethine ylide to activated alkene. J Org Chem 83:5392–5397. https://doi.org/10.1021/acs.joc.8b00085
Dong K, Xu X, Doyle MP (2020) Copper(i)-catalyzed highly enantioselective [3 + 3]-cycloaddition of γ-alkyl enoldiazoacetates with nitrones. Org Chem Front 7:1653–1657. https://doi.org/10.1039/D0QO00539H
Dorokhov VS, Golovanov IS, TartakovskySukhorukovIoffe VAAYSL (2018) Diastereoselective synthesis and profiling of bicyclic imidazolidinone derivatives bearing a difluoromethylated catechol unit as potent phosphodiesterase 4 inhibitors. Org Bio Chem 16:6900–6908. https://doi.org/10.1039/C8OB01039K
Gaonkar SL, Nagaraj VU, Nayak S (2019) A review on current synthetic strategies of oxazines. Mini-Rev Org Chem 16:43–58. https://doi.org/10.2174/1570193X15666180531092843
Gupta S, Khanna G, Khurana JM (2016) A facile eco-friendly approach for the one-pot synthesis of 3,4-dihydro-2H-naphtho[2,3-e][1,3]oxazine-5,10-diones using glycerol as a green media. Environ Chem Lett 14:559–564. https://doi.org/10.1007/s10311-016-0570-6
Hasegawa M, Suga T, Soeta T, Ukaji Y (2020) Synthesis of 3,6-Dihydro-2H-1,2-oxazines via dimethylsulfoxonium methylide addition to α, β-unsaturated nitrones. J Org Chem 85(17):11258–11264. https://doi.org/10.1021/acs.joc.0c01349
Huang X-R, Zhang Y-M, Wan T-B, Zhang P, Zhang X-X, Wang F-M, Xu D, Shen M-H, Xu H-D (2019) Straightforward synthesis of 4,5-bifunctionalized 1,2-oxazinanes via Lewis acid promoted regio- and stereo-selective nucleophilic ring-opening of 3,6-dihydro-1,2-oxazine oxides. Tetrahedron Lett 75:130336. https://doi.org/10.1016/j.tet.2019.05.015
Jadhav PD, Chen J-X, Liu R-S (2020) Gold(I)-catalyzed highly enantioselective [4 + 2]-annulations of cyclopentadienes with nitrosoarenes via nitroso-povarov versus oxidative nitroso-povarov reactions. ACS Catal 10:5840–5845. https://doi.org/10.1021/acscatal.0c01293
Jasinski M, Lentz D, Reissig H-U (2012) Carbohydrate-auxiliary assisted preparation of enantiopure 1,2-oxazine derivatives and aminopolyols. Beilstein J Org Chem 8:662–674. https://doi.org/10.3762/bjoc.8.74
Kumar P, Kumar R, Banerjee P (2020) Accessing dihydro-1,2-oxazine via cloke–wilson-type annulation of cyclopropyl carbonyls: application toward the diastereoselective synthesis of pyrrolo[1,2-b][1,2]oxazine. J Org Chem 85:6535–6550. https://doi.org/10.1021/acs.joc.0c00531
Kuzu B, Genç H, Taşpinar M, Tan M, Menges N (2018) An easy synthetic protocol for imidazo-1,4-oxazines and evaluation of their toxicities. Heteroatom Chem 29:21412. https://doi.org/10.1002/hc.21412
Li J, Yang F, Ma Y-T, Ji K (2019) Gold(III)-catalyzed intermolecular oxidation-cyclization of ynones: access to 4-substituted chroman-3-ones. Adv Synth Catal 361:2148–2153. https://doi.org/10.1002/adsc.201900260
Lin H, Sun X-W, Lin G-Q (2014) Dual-organocatalyst-promoted asymmetric cascade reaction: highly efficient construction of enantiopure fully substituted tetrahydro-1,2-oxazines. Org Lett 16:752–755. https://doi.org/10.1021/ol403463h
Liu F, Yu Y, Zhang J (2009) Highly substituted furo[3,4-d][1,2]oxazines: gold-catalyzed regiospecific and diastereoselective 1,3-dipolar cycloaddition of 2-(1-Alkynyl)-2-alken-1-ones with nitrones. Angew CHem INt Ed 121:5613–5616. https://doi.org/10.1002/ange.200901299
Ma G-L, Yang G-X, Xiong J, Cheng W-L, Cheng K-J, Hu J-F (2015) Salicifoxazines A and B, new cytotoxic tetrahydro-1,2-oxazine-containing tryptamine-derived alkaloids from the leaves of Chimonanthus salicifolius. Tetrahedron Lett 56:4071–4075. https://doi.org/10.1016/j.tetlet.2015.05.008
Manjula MK, Rai KML, Gaonkar SL, Raveesha KAS, Sathis S (2009) Synthesis of new series of 5,6-dihydro-4H-1,2-oxazines via hetero Diels-Alder reaction and evaluation of antimicrobial activity. Euro J Med Chem 44:280–288. https://doi.org/10.1016/j.ejmech.2008.02.027
Mokar BD, Liu J, Liu R-S (2018) Brønsted acids enable three molecular rearrangements of one 3-Alkylidene-2H-1,2-oxazine molecule into distinct heterocyles. Org Lett 16:1038–1041. https://doi.org/10.1021/acs.orglett.7b03985
Muratore ME, Konovalov AI, Relats HA, Echavarren AM (2018a) Diastereospecific gold(I)-catalyzed cyclization cascade for the controlled preparation of N- and N O-heterocycles. Chem Euro J 24:15613–15621. https://doi.org/10.1002/chem.201802770
Muratore ME, Konovalov AI, Relats HA (2018b) Diastereospecific gold(I)-catalyzed cyclization cascade for the controlled preparation of N- and N, O-heterocycles. Chem-Eur J 24:15613–15621. https://doi.org/10.1002/chem.201802770
Nguyen HV-T, Chen Q, Paletta JP, Harvey P, Jiang Y, Zhang H, Boska MD, Ottaviani MF, Jasanoff A, Rajca A, Johnson JA (2017) Nitroxide-based macromolecular contrast agents with unprecedented transverse relaxivity and stability for magnetic resonance imaging of tumors. ACS Cent Sci 3:800–811. https://doi.org/10.1021/acscentsci.7b00253
Rode ND, Arcadi A, Chiarini M, Marinelli F, Portalone G (2017) Gold-catalyzed synthesis of dibenzo[1,5]diazocines from β-(2-aminophenyl)-α, β-ynones. Adv Synth Catal 359:3371–3377. https://doi.org/10.1002/adsc.201700694
Roser P, Schmidt MJ, Drescher M, Summerer D (2016) Site-directed spin labeling of proteins for distance measurements in vitro and in cells. Org Biomol Chem 14:5468–5476. https://doi.org/10.1039/C6OB00473C
Santos JMI, Ignacio R, Rubiales G, Aparicio D, Palacios F (2011) Hetero-diels–alder reaction of phosphinyl and Phosphonyl nitroso alkenes with conjugated dienes: an Aza-cope rearrangement. J Org Chem 76(16):6715–6725. https://doi.org/10.1021/jo201116u
Sibi MP, Ma Z, Jasperse CP (2005) Enantioselective addition of nitrones to activated cyclopropanes. J Am Chem Soc 127:5764–5765. https://doi.org/10.1021/ja0421497
Taşdemir V, Menges N (2020) Gold-catalyzed cyclization of non-conjugated ynone-oxime derivatives: incorporation of solvent molecule. Asian J Org Chem 9:2108–2111. https://doi.org/10.1002/ajoc.202000415
Vatansever EC, Kılıç K, Özer MS, Koza G, Menges N, Balci M (2015) Intermolecular heterocyclization of alkynones with 2-mercaptoacetaldehyde under metal-free conditions: synthesis of 2,3-disubstituted thiophenes. Tetrahedron Lett 56:5386–5389. https://doi.org/10.1016/j.tetlet.2015.07.090
Wolinski PK, Zych A, Miroslaw B, Wielgus E, Olszewska A, Jasinski R (2022) Green, one-pot synthesis of 1,2-oxazine-type herbicides via non-catalyzed Hetero Diels-Alder reactions comprising (2E)-3-aryl-2-nitroprop-2-enenitriles. J Clean Prod 356:131878. https://doi.org/10.1016/j.jclepro.2022.131878
Xue H, Guo S, Hu T, Wei D, Xie Y, Shen J (2022) Synthesis and antiviral activity of 2’-deoxy-6’-substituted carbocyclic nucleosides. Chem Biol Drug Des 99:561–572. https://doi.org/10.1111/cbdd.13998
Yang B, Miller MJ (2010) Indium triflate-assisted nucleophilic aromatic substitution reactions of nitrosobezene-derived cycloadducts with alcohols. Org Lett 12:392–395. https://doi.org/10.1021/ol9027607
Yasukawa N, Kuwata M, Imai T, Monguchi Y, Sajiki H, Sawama Y (2018) Copper-catalyzed pyrrole synthesis from 3,6-dihydro-1,2-oxazines. Green Chem 20:4409–4413. https://doi.org/10.1039/C8GC01373J
Zhao J-R, Yuan X, Wang Z, Chen S, Zhang Z-X, Xue W (2015) Gold-catalyzed highly efficient benzylation of alcohols with N-Cbz-N-benzyl-propargylamine. Org Chem Front 2:34–37. https://doi.org/10.1039/C4QO00255E.f
Acknowledgements
This study was supported by the Turkish Academy of Sciences Outstanding Young Scientist Award (TÜBA-GEBİP). N.M. thanks TÜBA for their financial support. The authors thank YYU Science and Application Center for NMR and HRMS spectra.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
For this study, there is not any conflict of interest to be declared.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Taşdemir, V., Menges, N. Gold-catalyzed cyclization of unconjugated ynone derivatives for 2H-1,2-oxazine and 1-hydroxypyrrole skeletons through one-pot and single-step strategy. Chem. Pap. 77, 7985–7992 (2023). https://doi.org/10.1007/s11696-023-03041-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-023-03041-6