Abstract
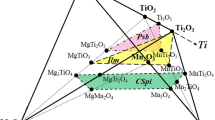
The isothermal section at 473 K of the Mg-Ni-Ga system in the entire composition regime and structural characterizations of the observed ternary phases are reported. The MgNi1+xGa1-x (x = 0.25), MgNiGa, Mg2NiGa3, Mg9-xNi6Ga14-y (x = 0.32, y = 0.84), Mg3Ni2Ga, and MgNi2Ga5 structures were solved and refined from X-ray single crystal diffraction data. MgNi1+xGa1-x (x = 0.25, Fd-3 m, a = 7.0781(2) Å) and MgNiGa (P63/mmc, a = 5.0781(3) Å, c = 8.194(1) Å) crystallize in the Laves phase MgCu2 and MgZn2 structure types, respectively. Mg2NiGa3 (Cmcm, a = 5.415(1) Å, b = 8.651(1) Å, c = 8.562(2) Å, Mg2MnGa3-type) represents the orthorhombic derivative of Laves phases. Mg9-xNi6Ga14-y (x = 0.32, y = 0.84, Fd-3 m, a = 19.8621(6) Å) is isostructural with Mg35Cu24Ga53. MgNi2Ga5 (Pnnm, a = 6.2704(1) Å b = 6.6902(1) Å c = 6.0794(1) Å) crystallizes in the MgCo2Ga5-type structure which is derived from tetragonal CoGa3-type. The crystal chemistry of these structures is compared and discussed. The hydrogenation properties of the MgNi1+xGa1-x (x = 0.25), MgNiGa, and Mg2NiGa3 Laves phases were studied. MgNi1.25Ga0.75 absorbs up to 2.20 wt.% H2, MgNiGa absorbs up to 1.78 wt.% H2, and Mg2NiGa3 absorbs up to 1.66 wt.% H2.






Similar content being viewed by others
References
M. Dornheim, S. Doppiu, G. Barkhordarian, U. Boesenberg, T. Klassen, O. Gutfleisch, and R. Bormann, Hydrogen Storage in Magnesium-Based Hydrides and Hydride Composites, Scr. Mater., 2007, 56, p 841–846.
A. Reiser, B. Bogdanovic, and K. Schlichte, The Application of Mg-Based Metal-Hydrides as Heat Energy Storage Systems, Int. J. Hydrog. Energy, 2000, 25, p 425–430.
G.S. Walker (2008) Multicomponent hydrogen storage systems, Solid-state Hydrogen Storage, p 478–499.
J.-C. Crivello, R.V. Denys, M. Dornheim, M. Felderhoff, D.M. Grant, J. Huot, T.R. Jensen, P. de Jongh, M. Latroche, G.S. Walker, C.J. Webb, and V.A. Yartys, Mg-Based Compounds for Hydrogen and Energy Storage, Appl. Phys. A, 2016, 122, p 85.
V. Pavlyuk, P. Solokha, O. Zelinska, V. Paul-Boncour, and A. Nowik-Zając, Ce20Mg19Zn81– a New Structure Type with a Giant Cubic Cell, Acta Cryst. C, 2008, 64, p 50–52.
P. Solokha, S. De Negri, V. Pavlyuk, and A. Saccone, Inhomogeneous 2D Linear Intergrowth Structures Among Novel Y-Cu–Mg Ternary Compounds With Yttrium/Copper Equiatomic Ratio, Solid State Sci., 2009, 11, p 801–811.
S. De Negri, P. Solokha, A. Saccone, and V. Pavlyuk, The YCuMg system in the 0667 at% Cu concentration range: The isothermal section at 400°C, Intermetallics, 2009, 17, p 614–621.
P. Solokha, S. De Negri, V. Pavlyuk, B. Eck, R. Dronskowski, and A. Saccone, 3D [Ag–Mg] Polyanionic Frameworks in the La4Ag10Mg3 and La4Ag10.3Mg12 New Ternary Compounds, Journal of Solid State Chemistry, 2010, 183(12), p 2995–3001. https://doi.org/10.1016/j.jssc.2010.10.018
G. Kowalczyk, V. Kordan, A. Stetskiv, and V. Pavlyuk, Lithiation and magnesiation of R5Sn3 (R = Y and Gd) alloys, Intermetallics, 2016, 70, p 53–60.
M.Y. Teslyuk, and V.Y. Markiv, New Ternary Laves Phases in Systems Containing Zn, Ga, In, and Ge, Sov. Phys. Crystallogr., 1962, 7, p 103–104.
N. Pavlyuk, G. Dmytriv, V. Pavlyuk, B. Rozdzynska-Kielbik, G. Cichowicz, M.K. Cyranski, I. Chumak, and H. Ehrenberg, New cubic cluster phases in the Mg–Ni–Ga system, Acta Cryst. B, 2020, 76, p 534–542.
N. Pavlyuk, G. Dmytriv, V. Pavlyuk, B. Rożdżyńska-Kiełbik, A. Gil, I. Chumak, H (2020) Ehrenberg, New ternary MgCo2Ga5 and MgNi2Ga5 gallides. Zeitschrift für Kristallographie-Crystalline Materials., 8,17.
G. Voss, Die Legierungen: Nickel-Zinn, Nickel-Blei, Nickel-Thallium, Nickel-Wismut, Nickel-Chrom, Nickel-Magnesium, Nickel-Zink und Nickel-Cadmium, Zeitschrift für Anorganische, Chemie., 1908, 57, p 34–71.
A.A. Nayeb-Hashemi, and J.B. Clark, Mg-Ni (Magnesium-Nickel) Binary Alloy Phase Diagrams, Second Edition, 1990, 3, p 2529–2530.
B. Predel, and D.W. Stein, Thermodynamische Untersuchung Des Systems Gallium-Magnesium, J.Less-Common Metals, 1969, 18, p 203–213.
A.A. Nayeb-Hashemi, and J.B. Clark, Ga-Mg (Gallium-Magnesium) Binary Alloy Phase Diagrams, Second Edition, 1990, 3, p 1822–1823.
S.Y. Lee, and P. Nash, Ga-Ni (Gallium-Nickel) Binary Alloy Phase Diagrams, Second Edition, 1990, 3, p 1829–1833.
P. Feschotte, and P. Eggimann, Les systemes binaires cobalt-gallium et nickel-gallium - étude comparée, Journal of the Less-Common Metals, 1979, 63, p 15–30.
J. Rodriguez-Carvajal, Recent Advances in Magnetic Structure Determination by Neutron Powder Diffraction, Physica B., 1993, 192, p 55–69.
Oxford Diffraction, CrysAlis CCD and CrysAlis RED, Oxford Diffraction Ltd., 2008.
APEX2, SAINT, XPREP and SADABS, Bruker AXS Inc., 2005.
G.M. Sheldrick, SHELXL-97. University of Göttingen, Program for crystal structure refinement, 1997.
O.K. Andersen, Linear methods in band theory, Phys Rev B., 1975, 12, p 30–60.
H. L. Skriver, The LMTO Method. Springer Berlin Heidelberg, Berlin, Heidelberg, 1984.
P. Phariseau, W.M. Temmerman, Eds., The Electronic Structure of Complex Systems Springer US, Boston, MA, 1984
O.K. Andersen, and O. Jepsen, Explicit, first-principles tight-binding theory, Phys Rev Lett., 1984, 53, p 2571–2574.
J. Korringa, On the calculation of the energy of a Bloch wave in a metal, Physica., 1947, 13, p 392–399.
W. Kohn, and N. Rostoker, Solution of the Schrödinger Equation in Periodic Lattices with an Application to Metallic Lithium, Phys Rev., 1954, 94, p 1111–1120.
G. Krier, O. Jepsen, A. Burkhardt, O.K. Andersen, The TB-LMTO-ASA program, version 4.7, Max-Planck-Institut für Festkörperforschung, Stuttgart, 1995.
U. von Barth, and L. Hedin, A local exchange-correlation potential for the spin polarized case, J. Phys. C Solid State., 1972, 5, p 1629–1642.
P.E. Blöchl, O. Jepsen, and O.K. Andersen, Improved tetrahedron method for Brillouin-zone integrations, Phys. Rev. B., 1994, 54, p 16223–16233.
R. Dronskowski, and P.E. Blöchl, Crystal orbital Hamilton populations (COHP): energy-resolved visualization of chemical bonding in solids based on density-functional calculations, J. Phys. Chem., 1993, 97, p 8617–8624.
A.D. Becke, and K.E. Edgecombe, A simple measure of electron localization in atomic and molecular systems, J. Chem. Phys., 1990, 92, p 5397–5403.
B. Silvi, and A. Savin, Classification of chemical bonds based on topological analysis of electron localization functions, Nature, 1994, 371, p 683–686.
B. Eck, wxDragon 1.6.6, Aachen, 1994–2010. Available from: http://www.ssc. rwth-aachen.de.
I. Chumak, V. Pavlyuk, V. Hlukhyy, and R. Pöttgen, Mg2MnGa3 – an orthorombic derivative of the hexagonal Laves-phase MgZn2, IX Inter. Conference on Crystal Chem. Intermetallic Compound., 2005, 20–24, p 51.
H. Bärnighausen, Group-subgroup relations between space groups: a useful Tool in Crystal Chemistry, Commun. Math. Comput. Chem., 1980, 9, p 139–175.
G. Cordier, and V. Müller, Crystal structure of Potassium Indium (17/41), K17In41, Z. Kristallogr., 1993, 205, p 353–354.
A. Chahine, M. Tillard-Charbonnel, and C. Belin, Crystal Structure of lithium copper gallium indium (18/5/31/4), Li18Cu5Ga31In4, Z. Kristallogr., 1995, 210, p 80.
P. Viklund, S. Lidin, P. Berastegui, and U. Häussermann, Variations of the FeGa3 structure type in the systems CoIn3−xZnx and CoGa3−xZnx, J. Solid State Chem., 2002, 165, p 100–110.
R. Pöttgen, R.-D. Hoffmann, and G. Kotzyba, Structure, chemical bonding, and properties of CoIn3, RhIn3, and IrIn3, Z. Anorg. Allg. Chem., 1998, 624, p 244.
E.I. Gladyshevskii, Yu.B. Kuz’ma, and P.I. Kripyakevich, The crystal structures of Mn3Ni2Si, V3Ni2Si, Nb3Ni2Si and related Cr and Ta compounds, J. Struct. Chem., 1963, 4, p 343–349.
Acknowledgements
Financial support from the National Science Centre, Poland (No 2017/25/B/ST8/02179) and German Academic Exchange Service (DAAD) is gratefully acknowledged (No 91774331, bilateral PhD scholarship grant).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pavlyuk, N., Dmytriv, G., Pavlyuk, V. et al. Mg-Ni-Ga System: Phase Diagram, Structural and Hydrogenation Properties of MgNi1.25Ga0.75, MgNiGa, and Mg2NiGa3. J. Phase Equilib. Diffus. 43, 458–470 (2022). https://doi.org/10.1007/s11669-022-00985-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-022-00985-2