Abstract
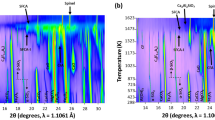
Complex silico-ferrites of calcium and aluminium (low-Fe form, denoted as SFCA; and high-Fe, low-Si form, denoted as SFCA-I) constitute up to 50 vol pct of the mineral composition of fluxed iron ore sinter. The reaction sequences involved in the formation of these two phases have been determined using an in-situ X-ray diffraction (XRD) technique. Experiments were carried out under partial vacuum over the temperature range of T=22 °C to 1215 °C (alumina-free compositions) and T=22 °C to 1260 °C (compositions containing 1 and 5 wt pct Al2O3) using synthetic mixtures of hematite (Fe2O3), calcite (CaCO3), quartz (SiO2), and gibbsite (Al(OH)3). The formation of SFCA and SFCA-I is dominated by solid-state reactions, mainly in the system CaO-Fe2O3. Initially, hematite reacts with lime (CaO) at low temperatures (T ∼ 750 °C to 780 °C) to form the calcium ferrite phase 2CaO·Fe2O3 (C2F). The C2F phase then reacts with hematite to produce CaO·Fe2O3 (CF). The breakdown temperature of C2F to produce the higher-Fe2O3 CF ferrite increases proportionately with the amount of alumina in the bulk sample. Quartz does not react with CaO and hematite, remaining essentially inert until SFCA and SFCA-I began to form at around T=1050 °C. In contrast to previous studies of SFCA formation, the current results show that both SFCA types form initially via a low-temperature solid-state reaction mechanism. The presence of alumina increases the stability range of both SFCA phase types, lowering the temperature at which they begin to form. Crystallization proceeds more rapidly after the calcium ferrites have melted at temperatures close to T=1200 °C and is also faster in the higher-alumina-containing systems.
Similar content being viewed by others
References
M. Sasaki and Y. Hida: Tetsu-to-Hagané, 1982, vol. 68, pp. 563–71 (in Japanese).
K. Kitamura, F. Shigemori, Y. Hatakeyama, T. Kawaguchi, and K. Takata: AIME Ironmaking Proc., 1985, vol. 44, pp. 405–14.
N.J. Bristow and A.G. Waters: Trans. Inst. Min. Metall. (Sect. C: Mineral Processing Extr. Metall.), 1991, vol. 100, pp. C1-C10.
I. Shigaki, M. Sawada, and N. Gennai: Trans. Iron Steel Inst. Jpn., 1986, vol. 26, pp. 503–11.
C.E. Loo, K.T. Wan, and V.R. Howes: Ironmaking and Steelmaking, 1988, vol. 15, pp. 279–85.
W.G. Mumme, J.M.F. Clout, and R.W. Gable: Neues Jahrbuch Miner. Abh, 1988, vol. 173, pp. 93–117.
SN. Ahsan, T. Mukherjee, and J.A. Whiteman: Ironmaking and Steelmaking, 1983, vol. 10, pp. 54–64.
P.R. Dawson, J. Ostwald, and K.M. Hayes: Trans. Inst. Min. Metall. (Sect. C: Mineral Processing Extr. Metall.), 1985, vol. 94, pp. C71-C78.
P.R. Dawson, J. Ostwald, and K.M. Hayes: BHP Tech. Bull., 1983, vol. 27, pp. 47–51.
T. Mukherjee and J.A. Whiteman: Ironmaking and Steelmaking 1985, vol. 12, pp. 151–56.
M.I. Pownceby and J.M.F. Clout: Trans. Inst. Min. Metall. (Sect. C: Mineral Processing Extr. Metall.), 2003, vol. 112, pp. C44-C51.
P.R. Dawson, J. Ostwald, and K.M. Hayes: Proc. Aus. Inst. Min. Metall., 1984, vol. 289, pp. 163–69.
Y. Hida, J. Okazaki, K. Itoh, and M. Sasaki: Tetsu-to-Hagané, 1987a, vol. 73, pp. 91–98 (in Japanese).
Y. Hida, M. Sasaki, K. Sato, M. Kagawa, M. Takeshi, H. Soma, H. Naito, and M. Taniguchi: Nippon Steel Tech. Rep., 1987b, vol. 35, pp. 59–67.
Y. Hida, T. Inazumi, K. Sato, and M. Sugata: Proc. 5th China-Japan Symp. on Science and Technology of Iron and Steel. International Academic Publishers, Beijing, 1989, pp. 96–107.
F. Matsuno: Trans. Iron Steel Inst. Jpn., 1979, vol. 19, pp. 595–604.
F. Matsuno and T. Harada: Trans. Iron Steel Inst. Jpn., 1981, vol. 21, pp. 318–325.
W.G. Mumme: Neues Jahrbuch Miner. Abh., 2003, vol. 178, pp. 307–335.
J.D.G. Hamilton, B.F. Hoskins, W.G. Mumme, W.E. Borbidge, and M.A. Montague: Neues Jahrbuch Miner. Abh., 1989, vol. 161, pp. 1–26.
Bruker AXS: TOPAS V2.1: General Profile and Structure Analysis Software for Powder Diffraction Data, Bruker AXS, Karlsruhe, Germany, 2003.
T.R.C. Patrick and M.I. Pownceby: Metall. Mater. Trans. B, 2002, vol. 33B, pp. 79–89.
B. Phillips and A. Muan: J. Am. Ceram. Soc., 1959, vol. 42, pp. 413–23.
M.I. Pownceby and T.R.C. Patrick: Eur. J. Mineralogy, 2000, vol. 12, pp. 455–68.
L.-H. Hsieh and J.A. Whiteman: Trans. Iron Steel Inst. Jpn., 1989a, vol. 29, pp. 24–32.
L.-H. Hsieh and J.A. Whiteman: Trans. Iron Steel Inst. Jpn., 1989b, vol. 29, pp. 625–34.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Scarlett, N.V.Y., Pownceby, M.I., Madsen, I.C. et al. Reaction sequences in the formation of silico-ferrites of calcium and aluminum in iron ore sinter. Metall Mater Trans B 35, 929–936 (2004). https://doi.org/10.1007/s11663-004-0087-4
Received:
Issue Date:
DOI: https://doi.org/10.1007/s11663-004-0087-4