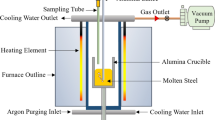
Abstract
There is a limit to lowering the nitrogen level in steel, because of pickup from the atmosphere during and after degassing. Therefore, the nitride capacity of various fluxes has been measured in order to examine the possibility of nitrogen removal by using slag. It is necessary to take into account the nitrogen reaction rate between the gas and slag phases for the efficient removal of nitrogen in practical steelmaking processes. In this study, the nitrogen desorption rate from CaO-Al2O3 melts to gas phase has been examined at 1873 K. The mass-transfer coefficient of nitrogen in the melts increases as the CaO content increases, and the dependence is related to the viscosity of the melts. The reaction-rate constant of nitrogen desorption is found to be proportional to the 3/2 power of the oxygen partial pressure and increases as the CaO content increases under constant oxygen partial pressure.
Similar content being viewed by others
References
K. Schwerdtfeger and H.G. Schubert: Metall. Trans. B, 1977, vol. 8B, pp. 535–40.
B. Ozturk and R.J. Fruehan: Ironmaking and Steelmaking, 1988, vol. 15, pp. 305–10.
K. Tomioka and H. Suito: Iron Steel Inst. Jpn. Int., 1991, vol. 31, pp. 1316–21.
H.O. Nakazato, D. Miyata, K. Tamura, and T. Usui: Iron Steel Inst. Jpn. Int. Suppl., 2000, vol. 40, pp. S106-S109.
H. Ono, K. Morita, and N. Sano: Metall. Mater. Trans. B, 1997, vol. 28B, pp. 633–38.
K. Iuchi, K. Morita, and N. Sano: Metall. Mater. Trans. B, 1998, vol. 29B, pp. 1235–40.
R.H. Perry and D.W. Green: Perry’s Chemical Engineer’s Handbook, 6th ed., McGraw-Hill, New York, NY, 1984, pp. 3–66.
A. Kikuchi, S. Taniguchi, T. Tadaki, and S. Maeda: Proc. Symp. Int. Union, Theoretical and Applied Mechanics, Cambridge, United Kingdom, 1982, The Metals Society, London, pp. 79–92.
R.B. Bird, W.E. Stewart, and E.N. Lightfoot: Transport Phenomena, John Wiley and Sons Inc., New York, NY, 1960, pp. 23, 513.
G.H. Geiger and D.R. Poirier: Transport Phenomena in Metallurgy, Addison-Wesley Publishing Co., New York, NY, 1973, p. 467.
T. Harada and D. Janke: Steel Res., 1989, vol. 60, pp. 337–42.
Y. Kawai and Y. Shiraishi: Handbook of Physico-Chemical Properties at High Temperatures, ISIJ, 1988, Tokyo, Japan, p. 121.
N.J. Themelis: Transport and Chemical Rate Phenomena, Gordon and Breach Publishers SA, 1995, Basel, Switzerland, pp. 257–78.
F. Tsukihashi, E. Oktay, and R.J. Fruehan: Metall. Trans. B, 1986, vol. 17B, pp. 541–45.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ono-Nakazato, H., Usui, T. & Morisawa, S. Rate of nitrogen desorption from CaO-Al2O3 melts to gas phase. Metall Mater Trans B 33, 393–401 (2002). https://doi.org/10.1007/s11663-002-0051-0
Received:
Issue Date:
DOI: https://doi.org/10.1007/s11663-002-0051-0