Abstract
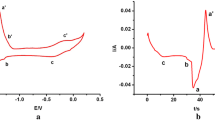
Cyclic voltammetry and square wave voltammetry were used to investigate reduction mechanism of Ti(IV) on the iron electrode surface in NaCl-KCl-NaF molten salt system. It indicated that the Ti(IV) ions were reduced to titanium metal by three steps, Ti(IV) → Ti(III) → Ti(II) → Ti, and the reduction process was irreversible process controlled by diffusion. The nucleation mechanism of titanium ions on pure iron electrode surface was determined to be an instantaneous nucleation process.









Similar content being viewed by others
References
Xu CY, Hua YX (2015) Electrochemical preparation of titanium and its alloy in ionic liquid. In: Handy S (ed) Ionic liquids—current state of the art. InTech, London, p 481–503
Liu R, Hui SX, Ye WJ et al (2013) Dynamic stress–strain properties of Ti–Al–V titanium alloys with various element contents. Rare Metals 32:555–559
Li J, Li B (2007) Electrochemical reduction and electrocrystallization process of Ti (IV) in the LiF-NaF-KF-K2TiF6 molten salt. Rare Metal Mater Eng 36:15–19
Haarberg GM, Kjos OS, Martinez AM et al (2010) Electrochemical behavior of dissolved titanium species in molten salts. ECS T 33:167–173
Bin W, Liu KR, Chen JS (2011) Reaction mechanism of preparation of titanium by electro-deoxidation in molten salt. Trans Nonferrous Metal Soc 21:2327–2331
Song Y, Jiao S, Hu L et al (2016) The cathodic behavior of Ti (III) ion in a NaCl-2CsCl melt. Metall Mater Trans B Process Metall Mater Process Sci 47:804–810
Malyshev V, Shakhnin D (2014) Titanium coating on carbon steel: direct-current and impulsive electro-deposition. Physicomechanical and chemical properties. Mater Sci 50:80–91
Li J, Zhang XY, Liu YB et al (2013) Electrochemical behavior of tungsten in (NaCl–KCl–NaF–WO3) molten salt. Rare Metals 32:512–517
Zhang M, Chen L, Han W et al (2012) Electrochemical behavior of Pb (II) in LiCl-KCl-MgCl2-PbCl2 melts on Mo electrode. Trans Nonferrous Metal Soc 22:711–716
Qian J, Tian Z (2012) Electrodeposition of iron platinum in NaCl-KCl molten salt. Trans Nonferrous Metal Soc 22:2855–2862
Li M, Li W, Han W et al (2014) Electrochemical behavior of Pr( III) in the LiCl-KCl melt on a Ni electrode. Chem J Chin Univ 35:2662–2667
Chen Z, Zhang ML, Han W et al (2008) Electrodeposition of Li and electrochemical formation of Mg–Li alloys from the eutectic LiCl–KCl. J Alloys Compd 459:209–214
Hu Y, Wang X, Xiao J et al (2013) Electrochemical behavior of silicon (IV) ion in BaF2-CaF2-SiO2 melts at 1573K. J Electrochem Soc 160:D81–D84
Liao CF, Fang MZ, Wang X et al (2015) Square wave voltammetry analysis of NaCl-KCl-Na2WO4 communion system. Nonferrous Metal Sci Eng 6:6–9
Xue Y, Wang Q, Yan YD et al (2013) Direct electrochemical reduction of Sm2O3 and formation of Al-Sm alloys in LiCl-KCl-AlCl3 melts. Chinese J Inorg Chem 29:1947–1951
Hu HL (2007) Electrochemical measurement. National Defense Industry Press, Beijing
Tang H, Yan YD, Zhang ML et al (2013) Electrochemistry of MgCl2 in LiCl-KCl eutectic melts. Acta Phys-Chim Sin 29:1698–1704
Hamel C, Chamelot P, Laplace A, Walle E, Dugne O, Taxil P (2007) Reduction process of uranium(IV) and uranium(III) in molten fluorides. Electrochim Acta 52:3995–4003
Gao P, Zhu YM (2013) Electrochemical basic tutorial. Chemical Industry Press, Beijing
Sun X, Lu G, Fan S (2014) Electrochemical mechanism of electrolysis co-deposition of Mg-Sr alloy in molten salt. Trans Nonferrous Metal Soc 24:1629–1634
Sun Y, Zhang M, Han W et al (2013) Electrochemical formation of Al-Li alloys by co-deposition of Al and Li from LiCl-KCl-AlF3 melts at 853 K. Chem Res Chin Univ 29:324–328
Zhang JQ (2010) Electrochemical measurement technology. Chemical Industry Press, Beijing
Allongue P, Souteyrand E (1990) Metal electrodeposition on semiconductors: part I. Comparison with glassy carbon in the case of platinum deposition. J Electroanal Chem Interfacial Electrochem 286:217–237
Funding
The study is financially supported by the National Natural Science Foundation of China (Project Nos. 51474093, 51401075), the Natural Science Foundation of Hebei Province (Project No. E2016209163), and the High School Science and Technology Research Project of Hebei Province (Project No. BJ2017050).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liang, J., Li, H., Huo, D. et al. Electrochemical characteristics of TiO2 in NaCl-KCl-NaF molten salt system. Ionics 24, 3221–3226 (2018). https://doi.org/10.1007/s11581-018-2503-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-018-2503-9