Abstract
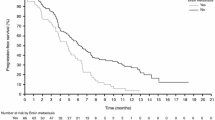
The BRAF inhibitor dabrafenib (Tafinlar®) and the MEK inhibitor trametinib (Mekinist®) are indicated, as monotherapy or in combination with each other, for the treatment of patients with unresectable or metastatic melanoma with a BRAF V600 mutation. This article reviews the therapeutic efficacy and tolerability of combination treatment with dabrafenib and trametinib in this indication and summarizes relevant pharmacological data. Dabrafenib plus trametinib significantly prolonged progression-free survival (PFS) and overall survival (OS), improved objective response rates (ORRs) and preserved health-related quality of life (HR-QOL) to a greater extent than dabrafenib (in the double-blind COMBI-d study) and vemurafenib (in the open-label COMBI-v study) in two large, randomized, phase III studies in treatment-naïve patients with unresectable or metastatic melanoma with BRAF V600E/K mutation. Limited treatment benefit with the combination was also seen in patients who had progressed on prior BRAF inhibitor therapy, as indicated by ORRs of ≤ 15 % and stable disease in ≤ 50 % of patients in small phase I and II studies. Combination therapy did not increase overall toxicity relative to dabrafenib or vemurafenib monotherapy, with most adverse events (AEs) mild or moderate in severity and generally manageable. Fewer skin-related AEs (e.g. cutaneous malignancies, hyperkeratinosis and hand-foot syndrome) were reported with combination therapy than with dabrafenib or vemurafenib, probably because of reduced paradoxical activation of the MAPK pathway. Thus, dabrafenib plus trametinib provides an important treatment option for patients with BRAF V600 mutation-positive unresectable or metastatic melanoma.


Similar content being viewed by others
References
Liu Y, Saeed SM. Melanoma: molecular pathogenesis and therapeutic management. Mol Cell Pharmacol. 2014;6(3):228.
World Health Organization. Ultraviolet radiation and the INTERSUN programme: skin cancers. 2016. http://www.who.int. Accessed 22 Apr 2016.
Olszanski AJ. Current and future roles of targeted therapy and immunotherapy in advanced melanoma. J Manag Care Spec Pharm. 2014;20(4):346–56.
John L, Cowey CL. The rapid emergence of novel therapeutics in advanced malignant melanoma. Dermatol Ther (Heidelb). 2015;5(3):151–69.
Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380(9839):358–65.
Holderfield M, Deuker MM, McCormick F, et al. Targeting RAF kinases for cancer therapy: BRAF-mutated melanoma and beyond. Nat Rev Cancer. 2014;14(7):455–67.
Paraiso KH, Fedorenko IV, Cantini LP, et al. Recovery of phospho-ERK activity allows melanoma cells to escape from BRAF inhibitor therapy. Br J Cancer. 2010;102(12):1724–30.
Su F, Viros A, Milagre C, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med. 2012;366(3):207–15.
GlaxoSmithKline. Tafinlar (dabrafenib): US prescribing information. 2015. http://www.pharma.us.novartis.com. Accessed 22 Apr 2016.
Novartis Europharm Ltd. Tafinlar (dabrafenib): summary of product characteristics. 2015. http://www.ema.europa.eu. Accessed 22 Apr 2016.
GlaxoSmithKline. Mekinist (trametinib): US prescribing information. 2015. http://www.pharma.us.novartis.com. Accessed 22 Apr 2016.
Novartis Europharm Ltd. Mekinist (trametinib): summary of product characteristics. 2016. http://www.ema.europa.eu. Accessed 22 Apr 2016.
King AJ, Arnone MR, Bleam MR, et al. Dabrafenib; preclinical characterization, increased efficacy when combined with trametinib, while BRAF/MEK tool combination reduced skin lesions. PLoS ONE. 2013;8(7), e67583.
Lito P, Pratilas CA, Joseph EW, et al. Relief of profound feedback inhibition of mitogenic signaling by RAF inhibitors attenuates their activity in BRAFV600E melanomas. Cancer Cell. 2012;22(5):668–82.
Gilmartin AG, Bleam MR, Groy A, et al. GSK1120212 (JTP-74057) is an inhibitor of MEK activity and activation with favorable pharmacokinetic properties for sustained in vivo pathway inhibition. Clin Cancer Res. 2011;17(5):989–1000.
Yoshida T, Kakegawa J, Yamaguchi T, et al. Identification and characterization of a novel chemotype MEK inhibitor able to alter the phosphorylation state of MEK1/2. Oncotarget. 2012;3(12):1533–45.
Schuchter LM, Kudchadkar RR, Gonzalez R, et al. Efficacy, safety, and pharmacokinetics (PK) of the BRAF inhibitor dabrafenib (D) hydroxypropyl methylcellulose (HPMC) capsule formulation in combination with the MEK1/2 inhibitor trametinib (T) in patients (pts) with BRAF mutation-positive metastatic melanoma (MM) [abstract no. 9066 plus poster]. J Clin Oncol. 2013;31(Suppl).
Ouellet D, Grossmann KF, Limentani G, et al. Effects of particle size, food, and capsule shell composition on the oral bioavailability of dabrafenib, a BRAF inhibitor, in patients with BRAF mutation-positive tumors. J Pharm Sci. 2013;102(9):3100–9.
Denton CL, Minthorn E, Carson SW, et al. Concomitant oral and intravenous pharmacokinetics of dabrafenib, a BRAF inhibitor, in patients with BRAF V600 mutation-positive solid tumors. J Clin Pharmacol. 2013;53(9):955–61.
Falchook GS, Long GV, Kurzrock R, et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet. 2012;379(9829):1893–901.
Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367(18):1694–703.
Johnson DB, Flaherty KT, Weber JS, et al. Combined BRAF (dabrafenib) and MEK inhibition (trametinib) in patients with BRAFV600-mutant melanoma experiencing progression with single-agent BRAF inhibitor. J Clin Oncol. 2014;32(33):3697–704.
Lawrence SK, Nguyen D, Bowen C, et al. The metabolic drug-drug interaction profile of dabrafenib: in vitro investigations and quantitative extrapolation of the P450-mediated DDI risk. Drug Metab Dispos. 2014;42(7):1180–90.
Bershas DA, Ouellet D, Mamaril-Fishman DB, et al. Metabolism and disposition of oral dabrafenib in cancer patients: proposed participation of aryl nitrogen in carbon-carbon bond cleavage via decarboxylation following enzymatic oxidation. Drug Metab Dispos. 2013;41(12):2215–24.
Ouellet D, Gibiansky E, Leonowens C, et al. Population pharmacokinetics of dabrafenib, a BRAF inhibitor: effect of dose, time, covariates, and relationship with its metabolites. J Clin Pharmacol. 2014;54(6):696–706.
Leonowens C, Pendry C, Bauman J, et al. Concomitant oral and intravenous pharmacokinetics of trametinib, a MEK inhibitor, in subjects with solid tumours. Br J Clin Pharmacol. 2014;78(3):524–32.
Infante JR, Fecher LA, Falchook GS, et al. Safety, pharmacokinetic, pharmacodynamic, and efficacy data for the oral MEK inhibitor trametinib: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13(8):773–81.
Cox DS, Papadopoulos K, Fang L, et al. Evaluation of the effects of food on the single-dose pharmacokinetics of trametinib, a first-in-class MEK inhibitor, in patients with cancer. J Clin Pharmacol. 2013;53(9):946–54.
US Center for Drug Evaluation and Research. Clinical pharmacology and biopharmaceutics review(s). 2013. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/204114Orig1s000ClinPharmR.pdf. Accessed 22 Apr 2016.
Ho MY, Morris MJ, Pirhalla JL, et al. Trametinib, a first-in-class oral MEK inhibitor mass balance study with limited enrollment of two male subjects with advanced cancers. Xenobiotica. 2014;44(4):352–68.
Ascierto PA, Minor D, Ribas A, et al. Phase II trial (BREAK-2) of the BRAF inhibitor dabrafenib (GSK2118436) in patients with metastatic melanoma. J Clin Oncol. 2013;31(26):3205–11.
Long GV, Trefzer U, Davies MA, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(11):1087–95.
Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367(2):107–14.
Kim KB, Kefford R, Pavlick AC, et al. Phase II study of the MEK1/MEK2 inhibitor trametinib in patients with metastatic BRAF-mutant cutaneous melanoma previously treated with or without a BRAF inhibitor. J Clin Oncol. 2013;31(4):482–9.
Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371(20):1877–88.
Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372(1):30–9.
McQuade JL, Chen G, Panka DJ, et al. Phase II study of dabrafenib and trametinib following progression on BRAF inhibitor monotherapy in metastaticmelanoma: exploration of clinical and molecular predictors of response [abstract no. e20051]. J Clin Oncol. 2015;33(Suppl).
Long GV, Weber JS, Infante JR, et al. Overall survival and durable responses in patients with BRAF V600-mutant metastatic melanoma receiving dabrafenib combined with trametinib. J Clin Oncol. 2016;34(8):871–8.
Long GV, Stroyakovskiy D, Gogas H, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled Trial. Lancet. 2015;386(9992):444–51.
Schadendorf D, Amonkar MM, Stroyakovskiy D, et al. Health-related quality of life impact in a randomised phase III study of the combination of dabrafenib and trametinib versus dabrafenib monotherapy in patients with BRAF V600 metastatic melanoma. Eur J Cancer. 2015;51(7):833–40.
Grob JJ, Amonkar MM, Karaszewska B, et al. Comparison of dabrafenib and trametinib combination therapy with vemurafenib monotherapy on health-related quality of life in patients with unresectable or metastatic cutaneous BRAF Val600-mutation-positive melanoma (COMBI-v): results of a phase 3, open-label, randomised trial. Lancet Oncol. 2015;16(13):1389–98.
Robert C, Karaszewska B, Schachter J, et al. Two year estimate of overall survival in COMBI-v, a randomized, open-label, phase III study comparing the combination of dabrafenib (D) and trametinib (T) with vemurafenib (Vem) as first-line therapy in patients (pts) with unresectable or metastatic BRAF V600E/K mutation-positive cutaneous melanoma [abstract no. 3301]. In: European Cancer Congress. 2015.
Heppt MV, Tietze JK, Graf SA, et al. Combination therapy of melanoma using kinase inhibitors. Curr Opin Oncol. 2015;27(2):134–40.
Larkin J, Ascierto PA, Dreno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371(20):1867–76.
National Comprehensive Cancer Network. NCCN clinical practice guidelines in Oncology: melanoma (Version 2.2016). 2016. http://www.nccn.org. Accessed 22 Apr 2016.
Macdonald JB, Macdonald B, Golitz LE, et al. Cutaneous adverse effects of targeted therapies: part II: inhibitors of intracellular molecular signaling pathways. J Am Acad Dermatol. 2015;72(2):221–36.
Gibney GT, Atkins MB. Immunotherapy or molecularly targeted therapy: what is the best initial treatment for stage IV BRAF-mutant melanoma? Clin Adv Hematol Oncol. 2015;13(7):451–8.
Hodi FS, Kluger H, Sznol M, et al. Durable, long-term survival in previously treated patients with advanced melanoma (MEL) who received nivolumab (NIVO) monotherapy in a phase I trial [abstract no. CT001]. In: American Association of Cancer Research annual meeting. 2016.
Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16(8):908–18.
Eroglu Z, Ribas A. Combination therapy with BRAF and MEK inhibitors for melanoma: latest evidence and place in therapy. Ther Adv Med Oncol. 2016;8(1):48–56.
Ascierto PA, Simeone E, Sileni VC, et al. Sequential treatment with ipilimumab and BRAF inhibitors in patients with metastatic melanoma: data from the Italian cohort of the ipilimumab expanded access program. Cancer Invest. 2014;32(4):144–9.
Ackerman A, Klein O, McDermott DF, et al. Outcomes of patients with metastatic melanoma treated with immunotherapy prior to or after BRAF inhibitors. Cancer. 2014;120(11):1695–701.
Dummer R, Hauschild A, Lindenblatt N, et al. Cutaneous melanoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26 Suppl 5:v126–32.
Kim T, Amaria RN, Spencer C, et al. Combining targeted therapy and immune checkpoint inhibitors in the treatment of metastatic melanoma. Cancer Biol Med. 2014;11(4):237–46.
National Institute for Health and Care Excellence. Trametinib in combination with dabrafenib for treating unresectable or metastatic melanoma: final appraisal determination. 2016. https://www.nice.org.uk/guidance/GID-TAG365/documents/final-appraisal-determination-document. Accessed 3 May 2016.
Long GV, Fung C, Menzies AM, et al. Increased MAPK reactivation in early resistance to dabrafenib/trametinib combination therapy of BRAF-mutant metastatic melanoma. Nat Commun. 2014;5:5694.
Acknowledgments
During the peer review process, the manufacturer of dabrafenib and trametinib was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of Interest
Sohita Dhillon is a salaried employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
The manuscript was reviewed by: M. A. Davies, Department of Melanoma Medical Oncology, Department of Systems Biology, Department of Translational Molecular Pathology, University of Texas MD Anderson Cancer Center, Houston (TX), USA; F. Grange, Department of Dermatology, Robert Debré University Hospital, Reims, France; P. Hersey, Sydney Medical School, University of Sydney, Sydney, Australia; P. Rutkowski, Department of Soft Tissue/Bone Sarcoma and Melanoma, Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland; R. J. Sullivan, Center for Melanoma, Massachusetts General Hospital Cancer Center, Boston (MA), USA.
Rights and permissions
About this article
Cite this article
Dhillon, S. Dabrafenib plus Trametinib: a Review in Advanced Melanoma with a BRAF V600 Mutation. Targ Oncol 11, 417–428 (2016). https://doi.org/10.1007/s11523-016-0443-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-016-0443-8