Abstract
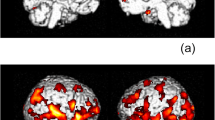
Despite the availability of combination antiretroviral therapy, at least mild cognitive dysfunction is commonly observed in HIV-infected patients, with an estimated prevalence of 35–70 %. Neuropsychological studies of these HIV-associated neurocognitive disorders (HAND) have documented aberrations across a broad range of functional domains, although the basic pathophysiology remains unresolved. Some of the most common findings have been deficits in fine motor control and reduced psychomotor speed, but to date no neuroimaging studies have evaluated basic motor control in HAND. In this study, we used magnetoencephalography (MEG) to evaluate the neurophysiological processes that underlie motor planning in older HIV-infected adults and a matched, uninfected control group. MEG is a noninvasive and direct measure of neural activity with good spatiotemporal precision. During the MEG recording, participants fixated on a central crosshair and performed a finger-tapping task with the dominant hand. All MEG data was corrected for head movements, preprocessed, and imaged in the time-frequency domain using beamforming methodology. All analyses focused on the pre-movement beta desynchronization, which is known to be an index of movement planning. Our results demonstrated that HIV-1-infected patients have deficient beta desynchronization relative to controls within the left/right precentral gyri, and the supplementary motor area. In contrast, HIV-infected persons showed abnormally strong beta responses compared to controls in the right dorsolateral prefrontal cortex and medial prefrontal areas. In addition, the amplitude of beta activity in the primary and supplementary motor areas correlated with scores on the Grooved Pegboard test in HIV-infected adults. These results demonstrate that primary motor and sensory regions may be particularly vulnerable to HIV-associated damage, and that prefrontal cortices may serve a compensatory role in maintaining motor performance levels in infected patients.




Similar content being viewed by others
References
Ances BM, Sisti D, Vaida F, Liang CL, Leontiev O, Perthen JE et al (2009) Resting cerebral blood flow: a potential biomarker of the effects of HIV in the brain. Neurology 73:702–708
Ances BM, Vaida F, Yeh MJ, Liang CL, Buxton RB, Letendre S et al (2010) HIV infection and aging independently affect brain function as measured by functional magnetic resonance imaging. J Infect Dis 201:336–340
Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M et al (2007) Updated research nosology for HIV-associated neurocognitive disorders. Neurology 69:1789–1799
Antiretroviral Therapy Cohort Collaboration (2008) Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet 372:293–299
Arendt G, Hefter H, Jablonowski H (1993) Acoustically evoked event-related potentials in HIV- associated dementia. Electroenceph Clin Neurophysiol 86:152–160
Becker JT, Bajo R, Fabrizio M, Sudre G, Cuesta P, Aizenstein HJ et al (2012a) Functional connectivity measured with magnetoencephalography identifies persons with HIV disease. Brain Imaging Behav 6(3):366–373
Becker JT, Fabrizio M, Sudre G, Haridis A, Ambrose T, Aizenstein HJ et al (2012b) Potential utility of resting-state magnetoencephalography as a biomarker of CNS abnormality in HIV disease. J Neurosci Methods 206(2):176–182
Chang L, Speck O, Miller EN, Braun J, Jovicich J, Koch C et al (2001) Neural correlates of attention and working memory deficits in HIV patients. Neurology 57:1001–1007
Chang L, Tomasi D, Yakupov R, Lozar C, Arnold S, Caparelli E, Ernst T (2004) Adaptation of the attention network in human immunodeficiency virus brain injury. Ann Neurol 56(2):259–272
Chang L, Yakupov R, Nakama H, Stokes B, Ernst T (2008) Antiretroviral treatment is associated with increased attentional load-dependent brain activation in HIV patients. J Neuroimmune Pharmacol 3:95–104
Cheney PD, Riazi M, Marcario JM (2008) Behavioral and neurophysiological hallmarks of simian immunodeficiency virus infection in macaque monkeys. J Neurovirol 14(4):301–308
Cheyne D, Bells S, Ferrari P, Gaetz W, Bostan AC (2008) Self-paced movements induce high- frequency gamma oscillations in primary motor cortex. NeuroImage 42:332–342
Cysique LA, Brew BJ (2009) Neuropsychological functioning and antiretroviral treatment in HIV/AIDS: a review. Neuropsychol Rev 19:169–185, Review
Deeks SG (2009) Immune dysfunction, inflammation, and accelerated aging in patients on antiretroviral therapy. Top HIV Med 17:118–123
Dore GJ, McDonald A, Li Y, Kaldor JM, Brew BJ, National HIV Surveillance Committee (2003) Marked improvement in survival following AIDS dementia complex in the era of highly active antiretroviral therapy. AIDS 17:1539–1545
Egan VG, Chiswick A, Brettle R, Goodwin G (1993) The Edinburgh cohort of HIV-positive drug users: the relationship between auditory P3 latency, cognitive function and self-related mood. Psychol Med 23:613–622
Ernst T, Chang L, Jovicich J, Ames N, Arnold S (2002) Abnormal brain activation on functional MRI in cognitively asymptomatic HIV patients. Neurology 59(9):1343–1349
Ernst T, Yakupov R, Nakama H, Crocket G, Cole M, Watters M et al (2009) Declined neural efficiency in cognitively stable human immunodeficiency virus patients. Ann Neurol 65:316–325
Fein G, Biggines CA, MacKay S (1995) Alcohol abuse and HIV infection have additive effects on frontal cortex function as measured by auditory evoked potential P3a latency. Biol Psychiat 37:183–195
Fox HS, Weed MR, Huitron-Resendiz S, Baig J, Horn TF, Dailey PJ et al (2000) Antiviral treatment normalizes neurophysiological but not movement abnormalities in simian immunodeficiency virus-infected monkeys. J Clin Invest 106(1):37–45
Gaetz W, Macdonald M, Cheyne D, Snead OC (2010) Neuromagnetic imaging of movement- related cortical oscillations in children and adults: age predicts post-movement beta rebound. NeuroImage 51:792–807
Gaetz W, Edgar JC, Wang DJ, Roberts TP (2011) Relating MEG measured motor cortical oscillations to resting γ-aminobutyric acid (GABA) concentration. NeuroImage 55:616–621
Gannon P, Khan MZ, Kolson DL (2011) Current understanding of HIV-associated neurocognitive disorders pathogenesis. Curr Opin Neurol 24:275–283, Review
Gelman BB, Lisinicchia JG, Chen T, Johnson KM, Jennings K, Freeman DH Jr, Soukup VM (2012) Prefrontal dopaminergic and enkephalinergic synaptic accommodation in HIV-associated neurocognitive disorders and encephalitis. J Neuroimmune Pharmacol 7(3):686–700
Goodin DS, Aminoff MJ, Chernoff DN, Hollander H (1990) Long latency event-related potentials in patients infected with human immunodeficiency virus. Ann Neurol 27:414–419
Gross J, Kujala J, Hamalainen M, Timmermann L, Schnitzler A, Salmelin R (2001) Dynamic imaging of coherent sources: studying neural interactions in the human brain. Proc Natl Acad Sci U S A 98:694–699
Hardy DJ, Vance DE (2009) The neuropsychology of HIV/AIDS in older adults. Neuropsychol Rev 19:263–272
Heaton RK, Clifford DB, Franklin DR Jr, Woods SP, Ake C, Vaida F et al (2010) HIV- associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 75:2087–2096
Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S et al (2011) HIV- associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 17:3–16
Hillebrand A, Singh KD, Holliday IE, Furlong PL, Barnes GR (2005) A new approach to neuroimaging with magnetoencephalography. Hum Brain Mapp 25:199–211
Hoechstetter K, Bornfleth H, Weckesser D, Ille N, Berg P, Scherg M (2004) BESA source coherence: a new method to study cortical oscillatory coupling. Brain Topogr 16:233–238
Janssen RS, Cornblath DR, Epstein LG, McArthur J, Price RW (1991) Nomenclature and research case definitions for neurological manifestations of human immunodeficiency virus type-1 (HIV-1) infection. Report of a Working Group of the American Academy of Neurology AIDS Task Force. Neurology 41:778–785
Johnson R (1988) The amplitude of the P300 component of the event-related potentials: review and synthesis. In: Ackles P, Jennings JR, Coles MGH (eds) Advances in psychophysiology: a research annual, 3rd edn. JAI Press Inc, Greenwich, pp 69–137
Joska JA, Gouse H, Paul RH, Stein DJ, Flisher AJ (2010) Does highly active antiretroviral therapy improve neurocognitive function? A systematic review. J Neurovirol 6:101–114, Review
Jurkiewicz MT, Gaetz WC, Bostan AC, Cheyne D (2006) Post-movement beta rebound is generated in motor cortex: evidence from neuromagnetic recordings. NeuroImage 32:1281–1289
Melrose RJ, Tinaz S, Castelo JM, Courtney MG, Stern CE (2008) Compromised fronto-striatal functioning in HIV: an fMRI investigation of semantic event sequencing. Behav Brain Res 188(2):337–347
Ollo C, Johnson R, Grafman J (1991) Signs of cognitive change in HIV disease: an event- related brain potential study. Neurology 41:209–215
Papp N, Ktonas P (1977) Critical evaluation of complex demodulation techniques for the quantification of bioelectrical activity. Biomed Sci Instrum 13:135–143
Polich J (1998) P300 clinical utility and control of variability. J Clin Neurophysiol 15:14–33
Polich J, Ehlers CL, Otis S, Mandell AJ, Bloom FE (1986) P300 latency reflects the degree of cognitive decline in dementing illness. Electroenceph Clin Neurophysiol 63:138–144
Polich J, Ilan A, Poceta JS, Mitler MM, Darko DF (2000) Neuroelectric assessment of HIV: EEG, ERP, and viral load. Int J Psychophysiol 38(1):97–108
Pollock VE, Schneider L, Lyness S (1990) EEG amplitudes in health, late middle-aged, and elderly adults: normality of the distributions and correlations with age. Electroenceph Clin Neurophysiol 75:276–288
Raymond LAM, Wallace D, Berman NEJ, Marcario J, Foresman L, Joag SV et al (1998) Auditory brainstem responses in a rhesus macaque model of neuro-AIDS. J Neuro Virol 4:512–520
Raymond LAM, Wallace D, Berman NEJ, Marcario J, Foresman L, Joag SV et al (1999) Motor evoked potentials in a rhesus macaque model of neuro-AIDS. J Neuro Virol 5:217–231
Raymond LAM, Wallace D, Zhou J, Raghavan R, Marcario JK, Johnson JK et al (2000) Sensory evoked potentials in SIV-infected monkeys with rapidly and slowly progressing disease. AIDS Res Hum Retroviruses 16:1163–1173
Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J et al (2007) The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS 21:1915–1921
Robertson K, Liner J, Heaton R (2009) Neuropsychological assessment of HIV-infected populations in international settings. Neuropsychol Rev 19:232–249
Sacktor N, McDermott MP, Marder K, Schifitto G, Selnes OA, McArthur JC et al (2002) HIV- associated cognitive impairment before and after the advent of combination therapy. J Neurovirol 8:136–142
Simioni S, Cavassini M, Annoni JM, Rimbault Abraham A, Bourquin I, Schiffer V et al (2010) Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS 24:1243–1250
Talairach G, Tournoux P (1988) Co-planar stereotaxic atlas of the human brain. Thieme, New York
Taulu S, Simola J (2006) Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys Med Biol 51:1759–1768
Taulu S, Simola J, Kajola M (2005) Applications of the signal space separation method (SSS). IEEE Trans Signal Process 53:3359–3372
The Mind Exchange Working Group (2013) Assessment, diagnosis, and treatment of HIV-associated neurocognitive disorder: a consensus report of the mind exchange program. Clin Infect Dis. [Epub ahead of print] PubMed PMID: 23175555
Thompson PM, Dutton RA, Hayashi KM, Toga AW, Lopez OL, Aizenstein HJ, Becker JT (2005) Thinning of the cerebral cortex visualized in HIV/AIDS reflects CD4+ T lymphocyte decline. Proc Natl Acad Sci U S A 102:15647–15652
Tozzi V, Balestra P, Bellagamba R, Corpolongo A, Salvatori MF, Visco-Comandini U et al (2007) Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: prevalence and risk factors. J Acquir Immune Defic Syndr 45:174–182
Tzagarakis C, Ince NF, Leuthold AC, Pellizzer G (2010) Beta-band activity during motor planning reflects response uncertainty. J Neurosci 30:11270–11277
van Veen BD, van Drongelen W, Yuchtman M, Suzuki A (1997) Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans Biomed Eng 44:867–880
Wilson TW, Slason E, Asherin RM, Kronberg E, Reite ML, Teale PD, Rojas DC (2010) An extended motor network generates beta and gamma oscillatory perturbations during development. Brain Cogn 73:75–84
Wilson TW, Slason E, Asherin RM, Kronberg E, Teale PD, Reite ML, Rojas DC (2011) Abnormal gamma and beta MEG activity during finger movements in early-onset psychosis. Dev Neuropsychol 36:596–613
Woods SP, Moore DJ, Weber E, Grant I (2009) Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychol Rev 19:152–168
Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, Winkler P (1997) Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain 120:141–157
Acknowledgments
This work was supported by NIH grant P30 MH062261 (HSF). The Center for Magnetoencephalography at the University of Nebraska Medical Center was founded through an endowment from an anonymous donor. We would like to thank our participants for volunteering.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wilson, T.W., Heinrichs-Graham, E., Robertson, K.R. et al. Functional Brain Abnormalities During Finger-Tapping in HIV-Infected Older Adults: A Magnetoencephalography Study. J Neuroimmune Pharmacol 8, 965–974 (2013). https://doi.org/10.1007/s11481-013-9477-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-013-9477-1