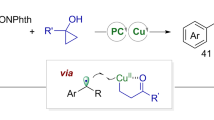
Abstract
Alkenes are ubiquitous, and the difunctionalization of alkenes represents one of the most practical approaches for the construction of value-added compounds. Dicarbonylation of alkenes provides direct access to value-added 1,4-dicarbonyl compounds. However, selectivity control for unsymmetric 1,2-dicarbonylation is of great challenge. We herein describe NHCs and photocatalysis co-catalyzed three-component radical 1,2-dicarbonylation of alkenes by distinguishing two carbonyl groups, providing structurally diversified 1,4-diketones. Distinct properties of acyl radical and NHCs-stabilized ketyl radical contributed to selectivity control. Acyl radicals are rapidly added to alkenes delivering alkyl radicals, which undergo subsequent radical-radical cross-coupling with NHCs-stabilized ketyl-type radicals, affording 1,2-dicarbonylation products. This transformation features mild reaction conditions, broad substrate scope, and excellent selectivity, providing a general and practical approach for the dicarbonylation of olefins.

Similar content being viewed by others
References
Wu X, Wu S, Zhu C. Tetrahedron Lett, 2018, 59: 1328–1336
Wu X, Zhu C. Acc Chem Res, 2020, 53: 1620–1636
Fischer H. Chem Rev, 2001, 101: 3581–3610
Leifert D, Studer A. Angew Chem Int Ed, 2020, 59: 74–108
Hartmann M, Li Y, Studer A. J Am Chem Soc, 2012, 134: 16516–16519
Li Y, Studer A. Angew Chem Int Ed, 2012, 51: 8221–8224
Speckmeier E, Fischer TG, Zeitler K. J Am Chem Soc, 2018, 140: 15353–15365
Beejapur HA, Zhang Q, Hu K, Zhu L, Wang J, Ye Z. ACS Catal, 2019, 9: 2777–2830
Zhu S, Qin J, Wang F, Li H, Chu L. Nat Commun, 2019, 10: 749–756
Patra T, Das M, Daniliuc CG, Glorius F. Nat Catal, 2021, 4: 54–61
Lai SQ, Wei BY, Wang JW, Yu W, Han B. Angew Chem Int Ed, 2021, 60: 21997–22003
Wang S, Tang S, Lei A. Sci Bull, 2018, 63: 1006–1009
Tang B, Zhao J, Xu JF, Zhang X. Chem Sci, 2020, 11: 1192–1204
Fürstner A, Nagano T. J Am Chem Soc, 2007, 129: 1906–1907
Renata H, Zhou Q, Dünstl G, Felding J, Merchant RR, Yeh CH, Baran PS. J Am Chem Soc, 2015, 137: 1330–1340
Li NS, Yu S, Kabalka GW. Organometallics, 1998, 17: 3815–3818
Yamamoto Y, Maekawa H, Goda S, Nishiguchi I. Org Lett, 2003, 5: 2755–2758
Liu Y, Zhang Y. Tetrahedron, 2003, 59: 8429–8437
Takaki K, Ohno A, Hino M, Shitaoka T, Komeyama K, Yoshida H. Chem Commun, 2014, 50: 12285–12288
Takaki K, Hino M, Ohno A, Komeyama K, Yoshida H, Fukuoka H. Beilstein J Org Chem, 2017, 13: 1816–1822
Zhao X, Li B, Xia W. Org Lett, 2020, 22: 1056–1061
Cheng YY, Yu JX, Lei T, Hou HY, Chen B, Tung CH, Wu LZ. Angew Chem Int Ed, 2021, 60: 26822–26828
Jin S, Sui X, Haug GC, Nguyen VD, Dang HT, Arman HD, Larionov OV. ACS Catal, 2022, 12: 285–294
Wang L, Sun J, Xia J, Li M, Zhang L, Ma R, Zheng G, Zhang Q. ChemRxiv, 2021, https://doi.org/10.26434/chemrxiv-2021-0c291
Liu J, Lu LQ, Luo Y, Zhao W, Sun PC, Jin W, Qi X, Cheng Y, Xiao WJ. ACS Catal, 2022, 12: 1879–1885
Chatgilialoglu C, Crich D, Komatsu M, Ryu I. Chem Rev, 1999, 99: 1991–2070
Enders D, Niemeier O, Henseler A. Chem Rev, 2008, 107: 5606–5655
Bugaut X, Glorius F. Chem Soc Rev, 2012, 41: 3511–3522
Flanigan DM, Romanov-Michailidis F, White NA, Rovis T. Chem Rev, 2015, 115: 9307–9387
Murauski KJR, Jaworski AA, Scheidt KA. Chem Soc Rev, 2018, 47: 1773–1782
Chen XY, Gao ZH, Ye S. Acc Chem Res, 2020, 53: 690–702
Bellotti P, Koy M, Hopkinson MN, Glorius F. Nat Rev Chem, 2021, 5: 711–725
Li T, Jin Z, Chi YR. Sci China Chem, 2022, 65: 210–223
Chen KQ, Sheng H, Liu Q, Shao PL, Chen XY. Sci China Chem, 2021, 64: 7–16
Sumida Y, Ohmiya H. Chem Soc Rev, 2021, 50: 6320–6332
Ishii T, Kakeno Y, Nagao K, Ohmiya H. J Am Chem Soc, 2019, 141: 3854–3858
Ishii T, Ota K, Nagao K, Ohmiya H. J Am Chem Soc, 2019, 141: 14073–14077
Li JL, Liu YQ, Zou WL, Zeng R, Zhang X, Liu Y, Han B, He Y, Leng HJ, Li QZ. Angew Chem Int Ed, 2020, 59: 1863–1870
Matsuki Y, Ohnishi N, Kakeno Y, Takemoto S, Ishii T, Nagao K, Ohmiya H. Nat Commun, 2021, 12: 3848
Kim I, Im H, Lee H, Hong S. Chem Sci, 2020, 11: 3192–3197
Mavroskoufis A, Jakob M, Hopkinson MN. ChemPhotoChem, 2020, 4: 5147–5153
Liu J, Xing X-N, Huang J-H, Lu L-Q, Xiao W-J. Chem Sci, 2020, 11: 10605–10613
Guin J, DeSarkar S, Grimme S, Studer A. Angew Chem Int Ed, 2008, 47: 8727–8730
Meng QY, Lezius L, Studer A. Nat Commun, 2021, 12: 2068
Meng QY, Döben N, Studer A. Angew Chem Int Ed, 2020, 59: 19956–19960
Liu K, Studer A. J Am Chem Soc, 2021, 143: 4903–4909
Zuo Z, Daniliuc CG, Studer A. Angew Chem Int Ed, 2021, 60: 25252–25257
Bay AV, Fitzpatrick KP, Betori RC, Scheidt KA. Angew Chem Int Ed, 2020, 59: 9143–9148
Bay AV, Fitzpatrick KP, González-Montiel GA, Farah AO, Cheong PHY, Scheidt KA. Angew Chem Int Ed, 2021, 60: 17925–17931
Ren SC, Lv WX, Yang X, Yan JL, Xu J, Wang FX, Hao L, Chai H, Jin Z, Chi YR. ACS Catal, 2021, 11: 2925–2934
Ren S-C, Yang X, Mondal B, Mou C, Tian W, Jin Z, Chi YR. Nat Commun, 2022, 13: 2846
Sato Y, Goto Y, Nakamura K, Miyamoto Y, Sumida Y, Ohmiya H. ACS Catal, 2021, 11: 12886–12892
Zhang B, Qi JQ, Liu Y, Li Z, Wang J. Org Lett, 2022, 24: 279–283
Penteado F, Lopes EF, Alves D, Perin G, Jacob RG, Lenardão EJ. Chem Rev, 2019, 119: 7113–7278
Zhang G, Liu Y, Zhao J, Li Y, Zhang Q. Sci China Chem, 2019, 62: 1476–1491
Wang L, Ma R, Sun J, Zheng G, Zhang Q. Chem Sci, 2022, 13: 3169–3175
Low yield of 42 by employing 87 as ketyl radical source might be caused by structural modification of the NHC
Zhuo J, Zhang Y, Li Z, Li C. ACS Catal, 2020, 10: 3895–3903
Kwon K, Simons RT, Nandakumar M, Roizen JL. Chem Rev, 2022, 122: 2353–2428
Huang H, Dai QS, Leng HJ, Li QZ, Yang SL, Tao YM, Zhang X, Qi T, Li JL. Chem Sci, 2022, 13: 2584–2590
Strieth-Kalthoff F, Glorius F. Chem, 2020, 6: 1888–1903
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22001157, 21831002, 22193012), the Ten Thousand Talents Program, the Fundamental Research Funds for the Central Universities (2412022QD016, 2412021QD007), the Natural Science Foundation of Jilin Province (YDZJ202201ZYTS338) and the Natural Science Foundation of Shaanxi Province (2020JQ-404).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest The authors declare no conflict of interest.
Additional information
Supporting information The supporting information is available online at https://chem.scichina.com and https://link.springer.com/journal/11426. The supporting materials are published as submitted, without typesetting or editing. The responsibility for scientific accuracy and content remains entirely with the authors.
Rights and permissions
About this article
Cite this article
Wang, L., Sun, J., Xia, J. et al. Visible light-mediated NHCs and photoredox co-catalyzed radical 1,2-dicarbonylation of alkenes for 1,4-diketones. Sci. China Chem. 65, 1938–1944 (2022). https://doi.org/10.1007/s11426-022-1328-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-022-1328-5