Abstract
Cardiovascular diseases (CVDs) have diverse physiopathological mechanisms with interconnected oxidative stress and inflammation as one of the common etiologies which result in the onset and development of atherosclerotic plaques. In this review, we illustrate this strong crosstalk between oxidative stress, inflammation, and CVD. Also, mitochondrial functions underlying this crosstalk, and various approaches for the prevention of redox/inflammatory biological impacts will be illustrated. In part, we focus on the laboratory biomarkers and physiological tests for the evaluation of oxidative stress status and inflammatory processes. The impact of a healthy lifestyle on CVD onset and development is displayed as well. Furthermore, the differences in oxidative stress and inflammation are related to genetic susceptibility to cardiovascular diseases and the variability in the assessment of CVDs risk between individuals; Omics technologies for measuring oxidative stress and inflammation will be explored. Finally, we display the oxidative stress-related microRNA and the functions of the redox basis of epigenetic modifications.
Similar content being viewed by others
Introduction
Cardiovascular diseases (CVDs) are considered as one of the leading reasons for global death (Chen et al. 2019). CVDs involve several pathologies such as disorders of the coronary and peripheral arteries as well as cerebrovascular diseases. Many of these diseases were related to atherosclerosis development (Choi et al. 2018). Furthermore, they may cause stroke, myocardial infarction (MI), and ischemia/reperfusion (I/R) injury. Other types of CVDs include atrial fibrillation (AF), chronic heart failure (CHF), and complications of cardiovascular diabetes. Dyslipidemia, smoking, hypertension, diabetes mellitus, and chronic kidney diseases (CKD) are well-known risk factors for CVDs which ultimately lead to oxidative stress and stimulate inflammation (Sárközy et al. 2018). Inflammation is one of the key intermediate pathways involved in the development of cardiovascular disorders (Koene et al. 2016).
In this regard, in hypertension, oxidative stress promotes endothelial dysfunction, vascular remodeling, and inflammation in hypertension which leads to vascular damage (Montezano et al. 2015). As inflammation is contributing to atherogenesis disease progression, anti-inflammatory treatments may offer a solution to cardiovascular problems (Welsh et al. 2017). Given the clear associations between high reactive oxygen species (ROS), oxidative stress, and heart disease, ROS has become an attractive treatment target to enhance cell antioxidant capabilities and ROS detoxification in several drug-based strategies (Peoples et al. 2019).
CVDs are a multicausal problem, in this regard, we focus on the causes related to inflammation and oxidative stress and how to manage them by considering risk factors, basic and advanced assessment, and treatment using healthy diet components and supplements. Challenges are also displayed with possible solutions. Methodology to find the article via PubMed last 10 years using keywords as MESH terms. Understanding the role of inflammation and oxidative stress is essential for increasing the chance of success during cardiovascular disease treatment. Moreover, new markers Exploration will also provide an opportunity to understand the mechanisms and new treatments for CVDs.
There are many basic laboratory markers (Tousoulis et al. 2012) and physiological tests (Lu et al. 2016) that help in the diagnosis and therapy of CVDs. Also, micro ribonucleic acids (miRNAs) are a type of non-coding RNAs that are intensively studied and have a significant impact on heart status by protein translation inhibition or target mRNA degradation so changes in their expression help in the diagnosis of CVDs (Dong et al. 2019). Besides, omics approaches such as redox transcriptomics, proteomics, and metabolomics as well as DNA methylation analysis could be used as tools for identifying clinical biomarkers; the integration of such data will increase cardiovascular disease understanding and speed up the identification of new diagnostics or therapeutic targets (Huertas-Vazquez et al. 2019).
In this paper, we will review the various CVDs risk factors that lead to oxidative stress and inflammation highlighting different cellular and molecular mechanisms with focusing on the assessment markers of oxidative stress status and inflammatory processes such as oxidative-stress-related microRNA as well as the redox basis of epigenetic modifications. Also, we will demonstrate the link between mitochondrial dysfunction and CVDs and how atherosclerotic plaques were formed. Furthermore, the mechanism of different antioxidant and anti-inflammatory agents to manage oxidative stress and inflammation with an overall description of other risk factors and their management.
Cellular and molecular mechanisms of oxidative stress and inflammation in CVDs
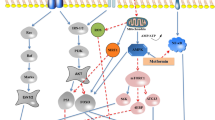
Nuclear factor erythroid 2 (Nrf2) is a pivotal CVD protection molecule that reduces oxidative stress, mitochondrial dysfunction, and inflammation. Following oxidative stress, Nrf2 is activated leading to its nuclear translocation and activation which subsequently regulates the transcription of a group of cardioprotective molecules such as hemeoxygenase-1 (HO-1), NADPH quinone oxidoreductase-1 (NQO1), and nuclear factor-κB (NF-κB). HO-1 expression is concomitant with glutathione S-transferases (GST) and superoxide dismutase (SOD) elevation leading to inhibition of oxidative stress (Uddin et al. 2020). NQO1 was known to regulate cardiac metabolism and reduce injury in inflammation with increasing resistance against oxidative stress, apoptosis, and atherosclerosis. Nuclear factor-kappa light chain-enhancer of activated B cells (NF-κB) restraining by Nrf2 can protect the heart and vasculature against injury (Jiang et al. 2016) as described in Fig. 1.
The pivotal role of nuclear factor erythroid 2 molecule in reducing oxidative stress, mitochondrial dysfunction, and inflammation which leads to CVDs protection. Nrf2: nuclear factor erythroid 2; HO- HO-1: hemeoxygenase-1; NQO: NADPH quinone oxidoreductase-1; NF-κB: nuclear factor-κB; GST: glutathione S-transferases; SOD: superoxide dismutase. ROS: reactive oxygen species; RNS: reactive nitrogen species
For the progression of CVDs, oxidative stress is considered as the key risk factor and is contributed to the excessive production of free radicals or ROS and the inability of cells to detoxify them (Powers et al. 2010), leading to a shift in the redox state of some organelles such as mitochondria and the nucleus. Subsequently, some macromolecules such as lipids, proteins, and DNA will be extremely damaged by oxidative stress (Choi et al. 2018) as shown in Fig. 1. ROS are usually formed during aerobic metabolism (Holterman et al. 2015). The most common ROS are hydroxyl radical (-.OH) and superoxide anions (O2.-) as well as hydrogen peroxide (H2O2) and considered the essential cellular redox status effectors (Loperena and Harrison 2017).
On the other hand, inflammation is another key significant risk factor for the pathological progression of CVDs (Choi et al. 2018). Two transcription factors in the inflammatory response pathways assist in CVD progression as key modulators: nuclear factor kappa light chain- enhancer of activated B cells (NF- κB) and hypoxia-inducible factor-1 alpha (HIF-1α) where the central inflammatory signaling cascade mediates by NF-κB (Nardinocchi et al. 2010). In the state of unstimulated cells, NF-κB heterodimer (p65/p50) is interacted with a class of inhibitory proteins called IκB (IκBα, -β, and -ε) and trapped in the cytosol so inflammation induction is inhibited. On the other hand, in the state of the stimulated cells, several stimuli enhance NF-κB activation, for instance, interleukin-1(IL-1), tumor necrosis factor-α (TNF-α), activators of protein kinase C (PKC), oxidants, and ionizing radiation. These stimuli induce phosphorylation and subsequent IκB protein degradation via the ubiquitin-proteasome system. Therefore, free NF-κB can translocate to the nucleus resulting in gene expression of pro-inflammatory genes such as chemokines, cytokines as well as adhesion molecules, which subsequently stimulate the inflammation process (Rao et al. 2017) as summarized in Fig. 2.
Many arterial wall pathological conditions are considered vascular diseases that may induce obstruction of blood flow. Such pathological conditions comprise atherosclerosis, arterial remodeling, restenosis, and thrombosis (Hulsmans and Holvoet 2010).
Atherosclerosis, the principal reason for CVD deaths, is a condition of chronic inflammation. Also, it is well known that the accumulation of lipids and/or fibrous materials and leucocytes in blood vessels and the arteries’ deepest layer is characteristic of atherosclerosis (Libby et al. 2010); this leads to the formation of fatty streak lesions called plaque in the vessel wall. Over time, an atherosclerotic plaque hardens and becomes more fibrous, and accumulates calcium mineral which results in the arteries narrowing and blood flow restriction. As a consequence, fatty plaques rupture, a blood clot, or a thrombus formed with an additional restriction of oxygen-rich blood flow to the body organs (Wiciński et al. 2020). Furthermore, the cumulative risk of atherogenic damage is increased by pathological disorders associated with prothrombotic, pro-inflammatory, and metabolic conditions (Clevenger 2016).
Oxidative stress does not only damage macromolecules directly and irreversibly but also disturbs the key redox-dependent signaling pathways of the arterial wall (Craige et al. 2015). In this context, nitric oxide (NO) was known to regulate cardiovascular homeostasis. The well-defined mechanism by which oxidative stress can enhance vascular disease may be through disturbance of the vasoprotection induced by NO signaling pathway (Murphy et al. 2016). NO is inactivated through reaction with superoxide anions and formation peroxynitrite (ONOO¯) leading to suppression of its anti-inflammatory and vasodilator functions (Stasch et al. 2011). Also, ONOO¯ is a powerful oxidant that causes oxidative stress by oxidation of antioxidants of small molecular size compounds such as tetrahydrobiopterin, cysteine, and glutathione (Stasch et al. 2011) (Fig. 3).
Besides, ONOO¯ can also oxidize and inhibit antioxidant enzymes, such as glutathione reductase, superoxide dismutase (SOD), and glutaredoxin (Stasch et al. 2011), as well as dimethylargininase-1 which metabolize asymmetric dimethylarginine, an endogenous endothelial NOS (eNOS) inhibitor (Murphy et al. 2016). Consequently, NO receptor dysfunction is caused by oxidative stress because of the binding of NO to the soluble guanylyl cyclase (sGC) haem group and further oxidation of its Fe2+ state to the Fe3+ state leading to a significant reduction in sGC affinity for NO (Stasch et al. 2011).
NO synthesis is catalyzed by nitric oxide synthase (NOS) three isoforms derived from the expression of discrete genes. The enzyme isoforms are called neuronal NOS (nNOS), and inducible NOS (iNOS), as well as endothelial NOS (eNOS) and, are encoded by NOS1, NOS2, and NOS3 genes respectively (Farah et al. 2018). It has been found that hypertension and endothelial dysfunction were caused by a decrease in the level of endothelial NO; this is in line with Coats and Jain study which indicated that the production of NO by nNOS regulates the release of catecholamine following the stimulation of the electrical adrenergic nerve, either in vitro or in vivo. Higher catecholamine levels are also related to the pathological condition of heart failure (Coats and Jain 2017). Besides, endothelial cells produce eNOS for NO generation after stimulation of muscarinic cholinergic receptors and adrenergic in the myocardium. It has been shown that acute and chronic β-adrenergic activation affects eNOS activity (Coats and Jain 2017) (see Fig. 4). eNOS enzyme activity is regulated by G-protein coupled receptors, modulators of AKT kinase, or calcium flux which are compartimentalized in caveolae or recruited to caveolae or nearby membrane regions upon activation (Heiss and Dirsch 2014).
Moreover, ROS may enhance vascular remodeling and inflammation. Most adverse effects of ROS on the arterial wall are attributed to CVDs (Kleniewska et al. 2012). It is well documented that the oxidation of major signaling phosphatases and kinases as well as the activation of NF-κB and subsequently stimulate the endothelial adhesion molecules expression. This in turn induced both proliferation and migration of vascular smooth muscle cells (VSMCs) which are the main cells in the arterial wall (Hai and Zuo 2016).
ROS also promotes the vascular remodeling by oxidation and subsequent activation of matrix metalloproteinases (MMPs) (Santillo et al. 2015) which are a group of zinc-dependent proteases that degrade many extracellular matrix (ECM) components and mediate remodeling in both physiological and pathological processes (Sampieri and Orozco-Ortega 2018). Most MMPs play a major role in the formation, remodeling, and angiogenesis of blood vessels by regulating the functions or behaviors of stem/progenitor and vascular cells (Chen et al. 2013).
The mitochondria play a key role in most cellular biology in eukaryotes. For example, in the cardiovascular system, it is mostly involved in both anabolic and catabolic metabolism as well as the initiation of the inflammatory reaction, regulation of intracellular Ca2+ homeostasis, and control of various pathways regarding regulated cell death (RCD) (Mehta et al. 2017). Furthermore, the mitochondrial system has been found to constantly undergo a tight quality control mechanism (Delbridge et al. 2017) known as mitophagy that is needed to optimally detect damaged mitochondria and delivering them to the lysosome for degradation (Harper et al. 2018).
In the crosstalk between mitochondrial function and redox biology, it is well known that ROS are normally produced from mitochondria during oxidative phosphorylation and physiological levels of ROS are involved in the regulation of various cardiovascular processes such as myocardial metabolic functions and vessel endothelial permeability (Shadel and Horvath 2015). Although mitochondrial dysfunction is generally linked to excessive production of ROS with low cellular antioxidant defenses, this will lead to oxidative damage to macromolecules resulting in local inflammation and initiation of many variants of RCD including the mitochondrial permeability transition driven (MPT) regulated necrosis and ferroptosis (Bonora et al. 2015). In agreement with these findings, it was showed that increasing ROS generation in the myocardium causes contractile failure as well as structural damage and ultimately leading to MI (Shirakawa et al. 2019).
In the presence of mitochondrial SOD2 and extramitochondrial SOD1, O2.- is converted into O2 and H2O2. Then, there are two fates for the metabolism of H2O2; the first one is catalyzed by catalase (CAT) results in the formation of H2O. The second fate was found to be catalyzed by glutathione peroxidase (GPx) which reduces H2O2 to water with the oxidation of reduced glutathione (GSH). Besides, in the presence of Cu1+ or Fe2+, H2O2 could be converted into OH• and OH– in the Fenton reaction (Bonora et al. 2019). Physiological ROS levels were found to control many biological processes such as autophagy, intracellular signaling, adaptation to hypoxia, and both adaptive and innate immunity (Sena and Chandel 2012). Nonetheless, ROS can cause extensive damage to macromolecules such as proteins, DNA, lipids when exceeding the capacities of endogenous antioxidants and subsequently results in RCD or cell senescence (Fig. 5).
NO controls cardiovascular function via two different mechanisms: the first one indirectly through increasing sGC activity and subsequent activation of its downstream substrate called protein kinase G, and the second one directly via the S-nitrosylation of proteins (Maron et al. 2013). Lipophilic NO properties prefer paracrine signaling by its migration from endothelial cells to cardiomyocytes, VSMCs, and vessel lumen, while autocrine signaling of NO takes place mostly in heart myocytes (Amelio et al. 2015). Hemoglobin-α (Hb-α) has been shown to regulate paracrine NO diffusion in the cardiac endothelial junction (Straub et al. 2012). The mechanism of endothelial Hb-α in NO signaling showed that Hb-α Fe3+ state induces this signaling and when converted to Hb-α Fe2+ by endothelial cytochrome b5 reductase 3, the NO signaling is inhibited. Therefore, the inhibition of endothelial cytochrome b5 reductase 3 could offer a therapeutic approach for increasing the bioactivity of NO in small arteries (Straub et al. 2012).
In the relationship between ROS and hypertension, oxidative stress stimulates vascular inflammation, endothelial dysfunction, enhanced functional, and structural remodeling causing increased blood pressure and peripheral resistance (Brito et al. 2015). NADPH oxidase (NOX) is transmembrane proteins with many subunits and in the membranes of smooth muscle cells, vascular endothelial cells, and fibroblasts, it induces the production of superoxide by transfer a single electron from NADPH to molecular O2, which belongs to ROS. Consequently, NOX enzymes have a crucial role in various CVDs pathophysiology (Touyz and Briones 2011).
Molecular processes regarding ROS affect hypertension development involves activation of vascular redox-sensitive signaling pathways, where the vasodilator NO is decreased with increased production of ROS. ROS (O2.- and H2O2) induce tyrosine kinases, mitogen-activated protein kinases (MAPK), Rho kinase, transcription factors including NF-κB, Activator protein-1 (AP-1), and HIF-1. Also, they suppress protein tyrosine phosphatases, increase intracellular free Ca2+ concentration, and increase proinflammatory and protooncogene gene expression as well as activity (Fig. 6) (Al Ghouleh et al. 2011). At the vascular level, these intracellular signaling alterations can subsequently result in endothelial dysfunction, decreased vasodilation, elevated contraction, and structural remodeling leading to enhanced peripheral resistance and hypertension (Touyz and Briones 2011). Maintaining normal O2 homeostasis via O2 sensing by the effect of ROS on HIF-1 regulation. In pathological conditions, ROS are involved in inflammation, endothelial dysfunction, cell activation, proliferation and migration, and extracellular matrix deposition, fibrosis, angiogenesis, and vascular remodeling (Gupta et al. 2016).
In the kidney, redox-sensitive pathway activation is implicated with glomerular damage, proteinuria, water and sodium retention, and nephron function loss which are crucial in the development of hypertension (Sasser et al. 2014). Centrally produced ROS by NADPH oxidase (NOX) in the hypothalamic and circumventricular organs are involved in central control of blood pressure elevation, partially through sympathetic outflow (Purushothaman et al. 2011). Although oxidative stress may involve the hypertension pathophysiology and damage of the associated target organ, it is not the sole cause of hypertension. The previous studies demonstrate that inhibitors of ROS bioavailability decline, but do not normalize the blood pressure in hypertension models (Montezano and Touyz 2014).
Risk factors for CVDs
Also, cardiovascular diseases are considered as both primary and secondary diseases, where many other disorders cause it, for instance, obesity, arterial hypertension, hypercholesterolemia, smoking, sedentary and an unhealthy lifestyle, aging, alcoholism, hyperglycemia, both type 1 and type 2 diabetes, and the metabolic syndrome (Fig. 7) (Rückert et al. 2012). Regarding vascular function, these metabolism disorders are viewed as significant risk factors in congestive heart failure and cardiac dysfunction progress (Rückert et al. 2012). Besides, CKD is a public health problem that accelerates the pathology of CVD (Sárközy et al. 2018) and it is well shown as the leading cause of morbidity and mortality in patients with CKD. The elevated incidence of CVD in CKD patients is only partially represented by the higher prevalence of traditional risk factors in these patients such as aging, dyslipidemia, hypertension, diabetes, smoking, and obesity (Subbiah et al. 2016) (Fig. 7).
The accumulation of uremic toxins in CKD including inorganic phosphate, para-cresyl sulfate, indoxyl sulfate, and fibroblast growth factor 23 (Six et al. 2020) is due to impairment of renal clearance (Nuhu and Bhandari 2018). Also, the uremic milieu comprises several cardiac risk factors that give respond to a distinct cardiac pathology termed uremic cardiomyopathy with the prevalence of left ventricular hypertrophy (Foley et al. 2010). The respiratory function of mitochondria in uraemia is affected by increased exposure to mitochondria toward calcium and stress-induced oxidation that may enhance the increased susceptibility of the uremic heart to I/R injury (Taylor et al. 2015).
Furthermore, the association between CKD and unique cardiomyopathy is characterized by both cellular and structural remodeling, and an increased risk of ischemia (Semple et al. 2012). Besides, the inflammation occurs by the accumulation of uremic toxins (Six et al. 2020) as well as oxidative stress, ROS induction of a decline in NO production (Ishigami et al. 2016), anemia (Pisani et al. 2015). This accumulation accelerates the pathology of CVD, such as heart failure, which directly causes kidney dysfunction. In considering the progression of CKD, part of the mechanism is via oxidant-induced damage to the glomerular basement membrane (Pisani et al. 2015). This complex association recognized cardiorenal syndrome (Guo et al. 2017). Patients with CKD are in a constant inflammatory case caused by several factors, including the uremic state, malnutrition, chronic volume overload, increased infection, metabolic acidosis, and autonomic dysfunction (Nuhu and Bhandari 2018).
Genetic composition which is concerned with the initiation and development of cardiovascular diseases can also be associated with oxidant stress (He and Zuo 2015). Regarding NOX, NOX1 and NOX2 were found to be associated with the induction of Angiotensin II for eNOS uncoupling with reduced tetrahydrobiopterin (BH4) levels (Sheehan et al. 2011). Also, angiotensin II causes vasoconstriction as well as an increase in both blood pressure, vascular muscle proliferation, and is involved in the ROS development cycle, resulting in vascular tissue damage (Kemp et al. 2014). Besides, NOX4 which is expressed by endothelial cells and VSMCs could produce H2O2 (Incalza et al. 2018) and subsequently increased activation of eNOS, 5- adenosine monophosphate-activated protein kinase-α, vascular endothelial growth factor (VEGF) receptor 2, and p38MAPK pathways which are vital for angiogenesis. p47phox is an essential NOX cytosolic subunit and its knockout causes a reduction in the generation of ROS, NO production via PI3K-AKT- eNOS (Mittal et al. 2014). The polymorphism of p22phox rs4673 has been established as a 1.53-fold risk for coronary artery disease (CAD) by affecting the p22phox protein function (Mazaheri et al. 2017).
nNOS (Gimbrone Jr and García-Cardeña 2016) and eNOS (Ponnuswamy et al. 2012) were found to protect from atherogenesis, whereas increased expression of iNOS leading to excessive NO production which participates in aggravating the development of atherosclerotic plaques (Förstermann et al. 2017).
The eNOS gene has been well investigated in three functional polymorphisms: the first is single nucleotide polymorphism (SNP) called 786 T > C (rs2070744) at 5′ flanking eNOS gene region was found to decline the activity of its gene promoter approximately by 50%, a missense mutation 894G >T (Glu298Asp, rs1799983) in exon 7 influences NO levels by affecting the activity of eNOS activity, and intron 4 a 27-bp VNTR (4b/a) polymorphism and is due to splicing of small interference RNA pre-mRNA processing of eNOS leading to suppression of the expression of eNOS (Group 2014). Also, three SNPs are significantly associated with CAD in ancestries of Middle Eastern, European, Asian, Asian–Indian, and African (Glu298Asp) (Rai et al. 2014). Glu298Asp and 4b / a are the strongest in the Middle East, while T786-C showed higher CAD risk among Asian subjects (Rai et al. 2014).
Regarding the myeloperoxidase (MPO) gene, two SNPs named -129G/A and -463G/A have been reported to influence transcription factor specificity protein1 (SP1) and thus affecting the expression of MPO (Mishra et al. 2011). Also, they have been shown to raise the risk for CAD by 1.94 and 1.53 respectively (Mishra et al. 2011). Furthermore, the incidence of hypertension increased by 1.31- and 1.69-fold with the xanthine oxidase (XO) SNPs, e.g., rs11904439 and rs148756340, respectively (Scheepers et al. 2016). Other XO SNPs have also increased hypertension in Japanese populations, i.e., 47686C > T, 69901A > C, and 67873A > C and 69901A > C was found to be substantially associated with atherosclerosis of the carotid artery (Johnson et al. 2018). Moreover, it has been documented that carriers of cyclooxygenase COX-2 rs5277 C-allele increased the risk for negative cerebrovascular and cardiac events in particular for CAD (Liu et al. 2017b). Also, the multivessel CAD incidence of the G-765C COX2 polymorphism (rs20417) has been reduced (Rostoff et al. 2014).
Also, in the promoter region of the arachidonate 5-lipoxygenase (ALOX5) gene, it has been shown that the SP1 addition/deletion polymorphism increases the risk for CAD by 4.47-folds via affecting low-density lipoprotein (LDL) and high-density lipoprotein (HDL) levels (Todur and Ashavaid 2012). The variants of ALOX15, i.e., rs2619112 GA and rs7217186 CT are increased CAD risk by 2.27- and 3.41-fold respectively (Kaur et al. 2018). Also, the haplotypes of 5-lipoxygenase-activating protein (ALOX5AP) including HapB and HapC haplotypes are found to raise the CAD risk by 1.67- and 2.41-folds, respectively (Stock et al. 2011). Moreover, the epistemic interactions of the ALOX5, ALOX5AP, and MPO were found to contribute to ischemic stroke synergistically (Liu et al. 2017a).
Plasma levels of SOD1 and SOD2 were reported to be increased in patients with CAD (Peng et al. 2016). The risk for cardiovascular disease is reportedly increased by the three SOD1 variants, called rs9974610, ars1041740, and rs10432782 (Neves et al. 2012). SOD2 V16A polymorphism raises the risk of cardiovascular disease, regardless of the glycaemic score, cholesterol, blood pressure, smoking, and age (Pourvali et al. 2016). In the presence of TT genotype, the risk for CAD was significantly associated with the SOD2 C24 T while the protective role of CC and TC genotypes was found (Pourvali et al. 2016). The polymorphism SOD3 R231G was found to impact the risk for CAD with the risk factors RG and GG genotypes leading to CAD severity and risk for MI risk by reducing the levels of α-tocopherol (Nozik-Grayck et al. 2016).
It has been documented that Pro198Leu polymorphism for GPX1 and GPX-1 activity of less than 23.9 U/g Hb leads to an increase in the risk for CAD by 2.14-fold, and 4.72- fold respectively (Wickremasinghe et al. 2016). Also, GPx1 activity was inversely associated with CAD severity and MI risk (Sies et al. 2017). The variation of GPx-1 in Pro198Leu (rs1050450 C / T) affects its activity because of the conformation of its active site structure (Klaunig et al. 2011). In diabetes mellitus type II, it has been found that both C198T GPx-1 and C609T NADP [1]: quinone oxidoreductase 1 variants participate in the risk for CAD (Ramprasath et al. 2012). Also, these variants can functionally influence the enzyme activities and in turn, the overall antioxidant capacity resulting in oxidative stress and subsequently CVD (Ramprasath et al. 2012).
Regarding heart disease, it has been well studied that a missense mutation named L55M (rs854560) affects Paraoxonase 1 (PON1) production and with the Q192R (rs662) missense mutation its catalytic efficacy is affected. The rs854560 “T-allele” results in high PON production via encoding methionine, while the one named “A-allele” decreases the activity of PON by encoding leucine. Previous studies show that AA (55LL) genotype is related to increased risk of insulin resistance, increased Intima-media thickness (IMT) carotid artery, hypertension, and increased lipoprotein involvement in the activity phospholipase A2, and thus the risk for CVDs (Ferretti et al. 2015). The genotype of PON1 named 192RR has higher enzyme activity that decreases in the following order: QQ > QR > RR, with almost extremely little PON activity in RR genotype contributing to coronary atherosclerosis (Lüersen et al. 2011).
Cigarette smoking causes the development and progression of atherosclerosis (Kunutsor et al. 2018) by stimulating endothelial dysfunction, alteration of lipid profile, and inducing inflammatory response and thrombosis (Messner and Bernhard 2014). Additionally, cigarette smoking was found to be associated with the development and progression of carotid atherosclerosis, probably partly via the inflammation pathway (Wang et al. 2018b).
In cigarette smoke, many components among 7000 chemicals are well known to mediate CVD pathophysiology (Talhout et al. 2011). Carbon monoxide, polycyclic aromatic hydrocarbons, nicotine, and heavy metals and their oxides are examples of toxic chemicals that have thorough effects on vascular endothelium, blood lipids, and clotting factors causing atherosclerosis which affects arteries (Roy et al. 2017). Also, smoking increased catecholamines release, which increased heart rate, vasoconstriction, and cardiac output as cardiovascular events (Martín-Timón et al. 2014). In short, atherogenesis starts when smoking-activated inflammatory cells bind to the endothelium weakened by smoking and accumulate below the surface of the vessel, causing chronic inflammation that promotes plaque production and growth by accumulating cells rich in cholesterol.
Other components are also involved in endothelial dysfunction, including heavy metals such as arsenic, lead, and mercury, which stimulate the oxidation of cellular proteins and may lead to structural cellular damage and endothelial dysfunction. Further endothelial dysfunction can be mediated by polycyclic aromatic hydrocarbons (Mudau et al. 2012). These compounds also promote the oxidation of LDL which is known as bad cholesterol and is proatherogenic as well as impairing the endothelial function (Mudau et al. 2012). Another lipid peroxidation product caused by tobacco is the lipoprotein acrolein, an aldehyde that reacts with HDL, making it inaccessible for the removal of cholesterol from vascular endothelium (Parthasarathy et al. 2010). This method destroys a significant mechanism used by the body to fight atherosclerosis.
Furthermore, smoking-induced thrombosis through elevated production of thrombosis factors such as thrombin, fibrinogen, and von Willebrand factor and decreased fibrinolysis, former is especially critical in CVD pathophysiology (Roy et al. 2017). Smoking is also related to increased insulin resistance and hyperinsulinemia that have been involved in diabetes and atherosclerosis acceleration (Audrain-McGovern and Benowitz 2011). Additionally, epigenetic changes of the body cells caused by cigarette smoke contribute to damage to vessel walls, thereby increasing the clotting and inflammation tendency and subsequently CVD (Vinci et al. 2013).
Furthermore, CVDs emerge from epigenetic aberrations and alterations (to improve or suppress gene expression). The epigenetic processes include methylation of DNA Cytosine–guanine-rich regions (CpG) by the action of DNA methyltransferases, post-translational histone modification performed by many enzymes such as histone methyltransferases, histone demethylases, histone acetyltransferases, and histone deacetylases (Katkam et al. 2019a).
The DNA methylation of cytosine at promoter sites is intended to downregulate expression by specifically blocking the binding of gene transcription factors thus altering the transcriptional machinery’s accessibility to DNA. DNA methyltransferases (DNMTs) such as DNMTs: DNMT1, DNMT3a, and 3b are known to catalyze DNA methylation by using S-adenosylmethionine (SAM) as a methyl donor. It has been observed that the biological processes associated with CVDs including inflammation, atherosclerosis, diabetes, and hypertension are regulated by differential methylation of the candidate genes (Duthie 2011). In CAD patients, the decreased levels of 5-methyltetrahydrofolate and SAM, and increased plasma homocysteine levels were recognized. Elevation of blood homocysteine levels was associated with decreased methylation of DNA in peripheral lymphocytes isolated from vascular disease patients (Handy et al. 2011). Also, in CAD patients, there is an increased DNA global methylation and serum homocysteine levels are higher than 12.5 μM (Milagro et al. 2013). These increased blood homocysteine levels have been shown to stimulate the expression of p66shc via the hypomethylation of its promoter resulting in increased oxidizing stress and decreased nitric oxide bioavailability (Kim et al. 2011). In the heart endothelium, the hypomethylation of eNOS is observed, while in the vascular smooth muscle cell line, it is hypermethylated. On the other hand, in response to inflammation caused by atherosclerotic plaque neointima, the expression of iNOS in most tissues is increased whereas methylation reduces its expression (Kim et al. 2013).
It has been shown that in post-translation, histones protruding N-terminal tails can be modified in a process called histone modifications (Kung et al. 2013). These modifications include methylation, acetylation, ubiquitination, phosphorylation, and sumoylation. Methylations at lysine 9, 27, and 36 on histone H3 caused a decreased gene expression, while methylations at lysine 4 and 79 on histone H3 and lysine 20 on histone H4 lead to an increased gene expression. These histone methylations are mediated through methyl group insertion by histone methyltransferases and methylation removal by histone demethylases. Acetylation of lysine on histones H3 and H4 induced an increase in gene transcription. The acetylation dynamics are also mediated by histone acetyltransferases which added acetyl groups and histone deacetylases which removed acetyl groups (Kaypee et al. 2016). In addition to affecting the transcription and gene expression, posttranslational histone modifications are also mediating apoptosis, and repair of DNA damage.
Regarding oxidative stress, it was found that alterations in cell metabolism and inflammatory responses lead to high levels of ROS and damaging DNA. In response to DNA repair, significant changes in surrounding chromatin including changes in nucleosome positioning and histone modifications are required (Vierbuchen et al. 2010). It has been shown that ROS and H2O2 induced epigenetic alterations, where after H2O2 treatment DNMT1 is more closely bound to chromatin and thus alters the methylation status of CpG regions (Tan et al. 2010). In another study, it has been shown that the upregulation of eNOS was associated with H3 and H4 histone increased acetylation in the eNOS promoter in neonatal rodent-persistent pH of the newborn model (Xu et al. 2010).
Damage to DNA sites caused by oxidative free radicals is identified by histone acetylases and deacetylases to promote repair and help improving repair protein access to the break site (Zhang et al. 2011). It has been recognized that a family of methyl-CpG binding proteins bind specifically to methylated CpGs, thus helping to repress transcription by recruiting histone-modifying proteins, including the methyl-CpG binding domain (MBD) protein family (MBD1, MBD2, MBD4, and MeCP2), Kaiso, and Kaiso-like proteins, and SRA domain proteins (e.g., SUVH9 and SUVH2) (Clapier et al. 2017). Histone methylation increases in caveolin knockout mice following I/R and is linked to the rise of histone deacetylases activity and the increase of the G9a histone methyltransferase protein level (Zhao et al. 2013).
The sirtuin (SIRT) family, consisting of seven proteins (SIRT1–SIRT7) sharing a strongly preserved catalytic domain of nicotinamide adenine dinucleotide (NAD+), acts as a stress adapter and epigenetic enzymes involved in the cellular events that regulate aging-related disease, cancer, and CVD (D'Onofrio et al. 2018). It was found that SIRT1, highly responsive to the cellular redox states, provides cardioprotection and vascular function maintenance by counteracting ROS effects via deacetylating multiple cellular targets (Vinciguerra et al. 2012). The redox role of the cells is regulated directly or indirectly by SIRT1, whose activity and expression can be affected by the cellular redox state by post-translation modifications (Hwang et al. 2013).
Via the deacetylation of eNOS at lysine (Lys)-496 and Lys-506, SIRT1 increases eNOS activity leading to increased production of NO, while SIRT1 knockdown has resulted in reduced production of NO and impaired endothelial-dependent vasodilation (Speakman and Mitchell 2011). At the vascular level, the physiological roles of SIRT1 and SIRT6 in the regulation of the cellular redox state are regulated by the deacetylation of multiple targets, including histones, transcription factors (FOXO, NF-kB, p53, and Nrf2), and vascular protective enzymes. SIRT6, a highly specific histone type 3 (H3) deacetylase, targets acetylated Lys-9 (acH3K9), Lys-56 (acH3K56), and Lys-18 (acH3K18). However, oxidative stress associated with various vascular dysfunctions impairs SIRT1 and SIRT6 activities on their specific targets, resulting in decreased vascular protection against oxidative stress (D'Onofrio et al. 2018).
SIRT1 and SIRT6 regulate inflammation by inhibiting the expression of inflammation-related genes, including ICAM-1, and proinflammatory cytokines, by deacetylating the p65 subunit of NF-kB. In endothelial cells, SIRT6 protects against senescence and oxidative stress by blocking the signaling of p21Cip1/Waf1 and maintaining high eNOS concentrations (D'Onofrio et al. 2018).
Basic and advanced approaches for oxidative stress and inflammation assessment in CVDs diagnosis
Biomarkers of oxidative stress status
It was reported that improving early CVD prediction is occurred by imaging (Fuster et al. 2010), genetic test (Pu et al. 2012), and biomarker assay (Tousoulis et al. 2012). The oxidative stress biomarkers are including malondialdehyde (MDA), conjugated dienes (CDs), hydroperoxides (R-OOH), F2-isoprostanes (F2-IsoPs), NOS family, MPO, uric acid (UA), protein carbonyl (PC), and trimethylamine N-oxide (TMAO) (Touyz and Briones 2011). MDA is produced from the arachidonate cycle and a principle aldehyde product of lipid peroxidation and is often measured as thiobarbituric acid (TBA) reactive substances (TBARS) (Lee et al. 2012), that is commonly used as a marker of oxidative stress in biological systems. Various studies reported that MDA and lipid hydroperoxide (LOOH) are increased in association with cardiovascular risk factors for instance diabetes mellitus and cigarette smoking (Lee et al. 2012).
Also, CDs produced because of free radical-induced autoxidation of polyunsaturated fatty acids (PUFAs) (Katerji et al. 2019), was measured as a marker of lipid peroxidation. The elevation of serum CD and TBARS levels of patients with CAD mediated by augmented production of lipid peroxidation (Speakman and Mitchell 2011). Besides, serum R-OOH as a product of lipid peroxidation induced by ROS molecules, this marker can be quantified by evaluating the derivatives of reactive oxygen metabolites assay, as described by Leufkens et al. (Leufkens et al. 2012). Also, hydroperoxides were generated from lipid oxidation and subsequently fragmented to produce many reactive intermediates, for example, prostaglandin F2α isomer F2-isoprostanes, and MDA (Nimse and Pal 2015). Experimentally and clinically, the measurement of LOOH was considered as a marker of peroxidative damage of membrane lipids and oxidative stress in vivo and they have a prognostic value in cardiovascular disease (Lee et al. 2012).
F2-IsoPs are a group of F2α-like prostaglandins, formed in vivo by non-enzymic arachidonic acid peroxidation esterified in phospholipids then hydrolyzed by platelet-activating factor acetylhydrolase to form their free acids. F2-IsoPs are chemically stable lipid peroxidation end products that are chemically stable compared to LOOH and MDA and can be detected in biological fluids, including urine, plasma, broncho-alveolar lavage, and cerebrospinal fluid as well as all human tissues (Rifkin et al. 2021). In the circulation, it was found that free F2-IsoPs are released from tissue then partially metabolized, and finally excreted into the urine. Besides, 8-Iso-prostaglandin (8-iso-PGF2α) is an abundant F2-IsoP formed in vivo in humans and has both platelet-activating and vasoconstrictive properties (Yin et al. 2011). Various studies reported that free and esterified F2-IsoPs in the plasma were significantly higher in smokers than non-smokers subjects and their levels significantly reduced after two weeks of smoking abstinence (Davies and Roberts II 2011). The level of F2-IsoPs was higher in adult subjects with high LDL or low HDL levels as well as in diabetic patients (Bouayed and Bohn 2010). Moreover, the urine F2-isoprostanes have a prognostic value in CVD, and both levels of urinary and plasma F2-IsoP levels were correlated with the number of CAD risk factors (Pompeani et al. 2014). Also, urinary 8-isoprostane was found to be a marker to known risk factors of coronary heart disease (CHD) such as smoking, diabetes mellitus, hypertension, and hypercholesterolemia (Dikalov and Ungvari 2013).
Moreover, the assessment of the NOS family is concerned with blood pressure regulation and cardiovascular function (Choi et al. 2018). NO functions as a signaling molecule that has an important role in vascular homeostasis. NO is a vasoactive substance synthesized from L-arginine by nNOS1, iNOS2, and eNOS3. eNOS3 is the vital NO synthase isoform in the vascular endothelium and consequently, it exerts critical roles in the cardiovascular system (Oliveira-Paula et al. 2017). Patients with cardiovascular risk factors (such as cigarette smoking, hypertension, hypercholesterolemia, diabetes mellitus, etc.) and patients with vascular disease show endothelial dysfunction, i.e., the failure of the endothelium to create an adequate amount of bioactive NO as well as delivery of NO-mediated vasodilation. Cardiovascular risk factors and vascular disease are also related to the enhanced production of ROS. ROS can be generated in the vessel wall by several enzymes such as xanthine oxidase, NOXs, enzymes of the mitochondrial respiratory chain, and uncoupled eNOS (Förstermann and Sessa 2012).
Also, MPO is an enzyme derived primarily from monocytes and neutrophils and plays a crucial role in leukocyte-mediated vascular injury responses. Such responses include oxidation of LDL, rendering it atherogenic and HDL, reducing its capacity to stimulate cholesterol efflux (Sugamura and Keaney Jr 2011). They are leading to a reduction in NO bioavailability, which causes endothelial dysfunction (Sugamura and Keaney Jr 2011). These effects confirmed that MPO is an actively mediated atherogenesis (Ho et al. 2013). Furthermore, in vascular disease animal studies, NOX was found to be activated in angiotensin-II induced hypertension (Lassègue et al. 2012), genetic hypertension (Förstermann et al. 2017), diabetes (Mittal et al. 2014) and hypercholesterolemia, and the expression of NOX subunits are potent in atherosclerotic arteries (Furukawa et al. 2017).
Also, it has been well documented that folate or one carbon atom pathway abolishes oxidative stress by their synergetic action with phase II enzymes of the xenobiotic metabolic pathway. Also, 5-methyltetrahydrofolate supplementation boosts the flow-mediated dilatation, an endothelial function marker (Yuyun et al. 2018). The one carbon atom metabolic pathway produces homocysteine which generates free radicals by autooxidation (Turell et al. 2013) and it has been documented that increased plasma homocysteine level is a risk factor for CAD (Ma et al. 2017).
Besides, PC content is considered as an indicator for the oxidation of proteins as well as a significant indication of oxidative stress in clinical trials in which protein backbones and amino acid residues such as threonine, lysine, proline, and arginine are oxidized by ROS molecules to generate PCs. Concerning the endogenous antioxidant biomarkers, they may be either enzymatic and non-enzymatic with the ability to eliminate ROS (Bonetta 2018) and maintain a cellular redox state. The non-enzymatic markers including peroxiredoxin (Prx), nicotinamide, metallothionein (MT); GSH. In response to animal tissue oxidative stress, the plasma GSH / GSSG ratio has also been reduced (Senoner and Dichtl 2019).
On the other side, the cell redox state is maintained by enzymatic markers such as glutathione-s-transferase (GST), (GPx), (CAT), and SOD (Bai et al. 2013) as well as heme oxygenase (HO), paraoxonase (PON), and the thioredoxin (TRX) system. Three forms of SODs were found in humans named SOD1, SOD2, and SOD3. While SOD3 is the major form in the human vascular wall and decreased superoxide concentrations by catalyzing the conversion of superoxide into hydrogen peroxide and oxygen molecule (Fatehi-Hassanabad et al. 2010). The CAT elevates the degradation of hydrogen peroxide to oxygen and water. In the preclinical model of atherosclerosis called ApoE mice, the upregulation of CAT and/or SOD expressions has been revealed to delay the atherosclerosis progression (Fatehi-Hassanabad et al. 2010). GPx has antioxidant effects by catalyzing the decrease of lipid peroxides and H2O2 to corresponding lipid alcohols and water via the oxidation of GSH into glutathione disulfide GSSG. Moreover, the activity of GPx isoform in red blood cells evaluated in CAD patients was shown to have a prognostic value (Sugamura and Keaney Jr 2011). Heme breakdown catalyzed by HOs to produce biliverdin that subsequently converted to bilirubin, which has an antioxidant effect and can inhibit NOX enzymes (Brandes et al. 2014).
It is well known that PON1 is a glycoprotein with antioxidant activity and its synthesis mainly takes place in the liver then it will be associated with HDL after its secretion in the blood (Meneses et al. 2019). PON1 is mainly involved in the protection of HDL and LDL against oxidative reactions and subsequently stopping its build-up or toxic products. Besides, PON1 stimulates macrophage cholesterol outflow from macrophages via HDL, reducing the differentiation between monocytes and macrophages and preventing the formation of atherosclerotic plaques (Kowalska et al. 2015).
The TRX system is a member of the oxidoreductase system existing in the VSMC and endothelial cells can scavenge ROS such as ONOO- and H2O2 (Sena et al. 2013). GSTs are a multigene family of isoenzymes that are extensively expressed in humans, as well as their catalytic role in the detoxification process by conjugating GSH to a variation of harmful electrophilic compounds (Katerji et al. 2019).
Furthermore, UA, the final component of the purine nucleotide metabolism is a significant human blood antioxidant and a marker of endothelial dysfunction and inflammation (Puddu et al. 2012). Although UA acts as a potent antioxidant in plasma, it boosts oxidative stress intracellularly. In vitro and in vivo studies have indicated that UA is one of the most selective antioxidants in the plasma because it can neutralize the risky pro-oxidants for example ONOO-, OH-, and iron-containing free radicals. Besides, it gives about 60% of the total plasma antioxidant capacity in humans (White et al. 2018). Serum UA rises, with almost 50% of patients starting hemodialysis being hyperuricemic (Kuo et al. 2013). Gathering indication refers that hyperuricemia is a risk factor for CKD that leads to renal failure (Johnson et al. 2013).
Measuring each antioxidant is complex and time-consuming and cost-effective. That is why the total antioxidant capacity (TAC) assessment in serum or plasma will provide clinicians with information about the patient’s antioxidant status as it reflects the complex interactions of antioxidants and their effect on the redox balance of the sample (Samaranayaka and Li-Chan 2011). There are many approaches used for measuring TAC (Rubio et al. 2016) and the given sample TAC value often depends on the procedure used in every specific assay. Ferric reducing the ability of serum is an established TAC estimating test, being an alteration of the ferric reducing ability assay of plasma (Lim and Lim 2013) generally utilized for TAC estimation. Additionally, the spectrophotometric 2.2-diphenyl-1-picryl-hydrazyl (DPPH) test has been reported as a more reliable way to measure TAC (Prymont-Przyminska et al. 2014). It was found that TAC is directly related to overweight/obesity indices as well as indices of metabolic syndrome laboratory measures, especially in coronary heart disease patients (Gawron-Skarbek et al. 2014).
In many studies, miRNAs were found to have important roles in cardiovascular development, pathology, repair, and regeneration and may be used to diagnose and prevent CVDs such as cardiac hypertrophy, CAD, I/R injury, and heart failure (Kreutzer et al. 2020; Kura et al. 2020). CVDs are induced and evolved by apoptosis, autophagy, necrosis, and fibrosis, as well as the proliferation and migration of cardiomyocytes, cardiac fibroblasts, endothelial cells, and VSMCs in response to increased ROS or stress stimuli. miRNAs have been reported to be employed in these processes (Colpaert and Calore 2019). Also, several miRNAs have been assigned in the cardiovascular system as regulators of oxidative stress by affecting ROS producers, antioxidant signaling mechanisms, and selected effectors of antioxidation (Gong et al. 2018).
For instance, in a study to examine whether hypercholesterolemia-induced myocardial microRNA alterations affect the production of oxidative or/ and nitrative stress and subsequent cardiac dysfunction, microRNA-25 demonstrated substantial downregulation as detected by microarray and QRT-PCR analysis. In rats fed with cholesterol, NOX4 protein was upregulated in the hearts and by downregulating microRNA-25, consequently oxidative/nitrative stress in the heart increases (Varga et al. 2013).
Intracellular ROS may also either inhibit or induce the level of miRNA expression and subsequently leading to biological effects by regulating their direct target genes (Banerjee et al. 2017). For example, multiple pathways (NF-κB, Nrf2, and SIRT1— sirtuin 1) were found to be closely associated with oxidative stress and miRNA (Kura et al. 2020) (Fig. 8).
Under oxidative stress, miRNAs affected different signaling mechanisms such as Nrf2, NF-κB, and SIRT1. ROS inhibits or induces the level of miRNA expression and subsequent their target genes regulation. The upper arrow reflects elevated oxidative stress/signaling pathway stimulation. Nrf2: nuclear factor erythroid 2; NF- κB: nuclear factor kappa-light chain- enhancer of activated B; SIRT1: sirtuin 1
miRNA could be a promising new approach in prognostic assessment, clinical diagnosis as well as oxidative-stress-related CVD therapy intervention. Understanding the crosstalk between miRNAs, ROS, and cardiovascular diseases that lead to new therapeutic approaches focused on suppressing the effects of ROS, with the potential for improvement or prevention of CVDs progression (Kura et al. 2020). Future miRNA studies may take advantage of numerous recent technological advances. High-throughput functional screening of miRNAs has increased the speed and accuracy of the discovery of new miRNAs associated with diseases (Fiedler et al. 2014). In vivo targeted delivery of miRNA is another technological advance predicted to move the field forward in the development of different animal models for studying miRNAs as well as enhancing the clinical translatability of pre-clinical studies (Kwekkeboom et al. 2014).
Biomarkers of inflammatory processes
NF-κB launches and boosts the inflammatory process via transcriptional activation of cellular adhesion molecules, cytokines, and chemokines (Brigelius-Flohé and Flohé 2011). This important pathological process plays a crucial role in the pathogenesis of several acute and chronic inflammation-related diseases for instance sepsis, asthma, and CVD (Oni-Orisan et al. 2013).
Regarding cardiovascular function and inflammation, it has been found that the expression of pro-inflammatory markers and cytokines (such as NF-κB, IL-1, IL-6, TNF-α, lymphotoxin, and interferon-γ (IFN-γ)) increased in vascular injury. Besides, C- reactive protein (CRP) which is an acute-phase protein is produced from the liver in response to tissue injury, inflammation, and infection (Brigelius-Flohé and Flohé 2011), and can act as a systemic inflammation biomarker (Goldstein et al. 2011). In atherosclerosis, C-reactive protein (CRP) is correlated with serum UA levels (Mazidi et al. 2018). Experimentally, it has been found that UA increases the release of chemokine monocyte chemoattractant protein-1 and IL-1β, IL-6, and TNF-α synthesis. Also, UA might contribute to atherosclerosis and the progression of human vascular disease through a pro-inflammatory pathway (Lyngdoh et al. 2011).
In the metabolic regulation of CRP, IL-6 is involved where chronic inflammation increases the levels of inflammation markers which result in high liver CRP in response to IL-6, which mediates a vasodilation reduction and vascular damage increase (Teixeira et al. 2014). Subsequently, NO production is decreased by high levels of serum IL-6, and CRP via suppressing eNOS and facilitating thrombi formation (Teixeira et al. 2014). IL-1β, IL-6, and TNF-α have also been found to be elevated in metabolic syndrome subjects (Koçak et al. 2016). Therefore, cytokines are useful biomarkers or targets for atherosclerosis therapy as they are increasing their pathogenicity. This could be due to their involvement in pro-inflammatory and pro-angiogenic responses (Zhao 2018).
Also, fibrinogen has been recognized as a marker of thrombosis as well as inflammation and was associated with increased CVD risk (Sabater-Lleal et al. 2013). Eicosanoids are biologically active fatty acids derived through oxidative metabolism of arachidonic acid by cyclooxygenase (COX), lipoxygenase (LOX), and cytochrome P450 enzyme systems (Mozaffarian and Wu 2011). COX is known to induce the conversion of the arachidonic acid into hydroperoxy-endoperoxide, prostaglandin G2, which is reduced to the precursor for eicosanoid synthesis called hydroxyl-endoperoxide prostaglandin H2 (Yamaguchi and Fukasawa 2021). The expression of COX2 and eicosanoids production, especially in macrophages and foam cells, is augmented in atherosclerotic lesions (Ricciotti and FitzGerald 2011). Also, COX-derived prostaglandins and biosynthesis of prostaglandins are important regulators of inflammation, so the inhibition of this reaction is considered as potent anti-inflammatory effects in preclinical models and humans (Ricciotti and FitzGerald 2011). Also, it has been shown that the metabolism of arachidonic acid to pro-inflammatory leukotrienes is catalyzed by the LOX pathway. Pro-inflammatory leukotrienes are known to induce vasoconstriction and elevate atherosclerosis risk (Katkam et al. 2019b). It was observed that ALOX5 knockout induced resistance against atherosclerosis progress in animal models (Sugamura and Keaney Jr 2011). In advanced plaques, ALOX5 levels were increased (Bhattacharyya et al. 2014). Most notably, prostaglandin E2 and thromboxane A2 are key pro-inflammatory products of this pathway that activate NF-κB, promote leukocyte infiltration into the tissue, and thus drive the vascular inflammatory response (Brooks et al. 2018).
Physiological tests for CVD prediction
The mechanisms of cardiovascular system multiphysics mechanisms can be detected by cardiac physiological tests. One of these tests is blood pressure which reflects the artery's hemodynamics. High blood pressure causes atherosclerosis development and it is common in strokes or heart attacks (Daugherty et al. 2017). Also, for screening vulnerable myocardium resulting in acute MI, an electrocardiogram (ECG) has been used as it assesses the heart electrophysiology and its abnormalities (Stone et al. 2011). Furthermore, arterial stiffness measurement by pulse wave analysis or aortic pulse wave velocity indicates the arteries’ blood fluid flow and the arterial wall hardening degree (Members et al. 2010) which consequently leads to arteriosclerosis. Another test is the index for ankle-brachial blood pressure which is the ratio between ankle and arm systolic pressure and assessed blood vessel structure and function as well as peripheral vascular disease. Measuring. IMT is another marker of early atherosclerosis and predicts CVD progress (Libby et al. 2011).
Omics technologies to assess oxidative stress and inflammation: promises and difficulties to overcome
The omics technologies show changes in the disease-related DNA, RNA, epigenetics, proteins, and metabolism. When oxidative stress is known to contribute to the development of a disease, genomic markers could be provided for this imbalance by the identification of specific disease mutations (Lowe 2014). The process of biomarker discovery can be considerably accelerated via omics technology, particularly by the combining of genomics, transcriptomics, proteomics, and metabolomics in a multi-omics strategy (Lee et al. 2018). In the next part, we will review the main components of the cellular redox response system utilizing “omics.”
Redox transcriptome
In response to oxidant stress, mammalian cells induced signaling cascades which can trigger the expression of a broad range of protein-coding genes that control the cellular redox status (Ma 2010). Noncoding RNAs contribute to redox gene expression regulation as well as protein-coding genes (Giannakakis et al. 2015). Genome-wide transcriptional profiling may also help predict oxidative stress biomarkers or related illnesses (Wang et al. 2018a). The findings of Genome-Wide Association Study (GWAS) on risk loci in a wide cohort of the population indicate a significant impact of arterial wall-specific inflammatory pathways in the CAD development and progression; for example, SNPs in or near genes associated with cellular adhesion, leukocyte migration and atherosclerosis (platelet endothelial cell adhesion molecule-1, rs1867624), and coagulation and inflammation (endothelial protein C receptor, rs867186 (p.Ser219Gly) were recognized (Howson et al. 2017). Also, certain GWAS data included several risk loci that are associated with cardiovascular inflammation (Klarin et al. 2017).
Furthermore, chromatin immunoprecipitation (ChIP)-Seq combines ChIP with massively parallel DNA sequencing and can integrate detection of genome-wide protein–DNA binding events (transcription factor-gene interaction) with chemical modifications of histone proteins (Furey 2012). Utilizing a combination of ChIP-Seq and microarray analyses provides a valuable method to identify upstream regulatory factors that control gene transcription. Through this approach, novel targets of the Nrf2 antioxidant transcription factor have been identified, providing a picture of a global transcriptional network regulated by Nrf2 (Chorley et al. 2012).
Redox proteomics
It is a branch of proteomics that aims at detecting oxidized proteins and quantifying the status of oxidative changes in the proteomes of interest. Such quantitative information is important for the building of mathematical models to decode the dynamics of redox associated with biochemical pathways and networks. In this regard, there have been studies focusing on the development of proteomics techniques to identify all redox-regulated proteins and redox-active compounds and to elucidate redox control networks in cellular systems (Buettner et al. 2013).
Fedorova and colleagues carried out a successful study using large-scale omics data in redox biology by applying a multi-omics approach to classify crosstalk signaling between lipid and protein carbonylation in rat cardiomyocytes by using the dynamic nitrosative stress model (Griesser et al. 2017). Lipidomics and proteomics system biological integration allowed over 167 unique proteins to be identified with 332 sites altered via reactive lipid peroxidation products. Although redox proteomics is still in its early stages, mass spectrometry (MS) driven biomarkers are a promising strategy in diseases related to oxidative and nitrosative stress. Redox proteomics is becoming a key tool for new insights into several disease-related protein modifications. Clinical research by recognizing new drug targets and diagnostic markers can benefit from redox proteomics (Wang et al. 2018a).
Redox metabolome
Metabolomics is one of the most groundbreaking medical technologies and is also known as metabonomics. By chemical analysis of a wide variety of biological sample metabolites, such as urine and blood, offers a direct functional read-out of phenotypes. Metabolites (< 1500 Da) reflect the cellular metabolism output, account for the activity and expression of genes, transcripts, and proteins, and offer unique insights into small molecule regulation, which may reveal new biochemical patterns. The redox metabolome is a metabolome redox-active subset. Redox and nitrosative (RN) reactions play a major role in CVD development and progression. The homeostatic regulation of a wide range of cellular and organ functions usually includes RN reactions. Conversely, allostasis which can cause CVD may result from the imbalance of these reactions (Deidda et al. 2018).
Nuclear magnetic resonance (NMR) and liquid chromatography (LC) or gas chromatography, combined with MS, are two main analytical methods for global metabolomic profiling. They can be applied to measure the composition and concentration of both specific and non-target metabolites in disease diagnosis and biochemical pathways analysis. The redox metabolic profiling techniques can be used to recognize metabolic biomarkers and to detect redox-regulated signaling mechanisms (Wang et al. 2018a).
DNA methylation analysis
DNA methylation takes place through the covalent addition of a methyl group at the five’ carbon of the cytosine ring, which forms 5-methylcytosine. The mammalian gene promoter regions which regulate the associated gene expression usually have cytosine–guanine-rich regions (CpG islands) in their genetic sequence. Methylation at specific sites can prevent the binding of transcriptional machinery or ubiquitous transcription factors to regulatory sites on the DNA double helix (Stone et al. 2011). The subsequent alteration in chromatin structure renders the promoter sequence of the target DNA inaccessible to activating transcription factors and inhibits gene expression.
Methylation controls gene activity, but without histone deacetylation and chromatin-binding proteins, it is not enough to repress the gene activity. The subsequent regulation of gene expression may lead to various disease processes involving oxidative stress (Giannakakis et al. 2015). Moreover, eicosapentaenoic acid allows a systematic evaluation of numerous CpG sites across the genome concerning the phenotype or disease of interest (Soler-Botija et al. 2019). The prediction of different drug responses can be assisted by epigenetic knowledge. Particularly in the scientific community, epigenetic biomarkers become identified as tools for the diagnosis and prognosis of CVDs (Soler-Botija et al. 2019).
While omics technologies have a wide range of applications in various fields, current challenges and technique limitations are present. The sensitivity and resolution of omics instruments have constantly been an issue in the field. For instance, only highly abundant proteins can be detected by some proteomics techniques due to insufficient sensitivity for low abundance proteins. Also, measurement and monitoring of the redox proteome have not achieved high resolution. Also, RNA-Seq examines mRNA sequence data from individual cells and performs accurate quantitative transcriptome measurements with greater cellular differentiation resolution than currently proteomic methods. Also, given the heterogeneity of responses across various cells, new techniques are needed to revise the redox transcriptome (Wang et al. 2018a).
Regarding proteomics and metabolomics studies, sample preparation often eliminates the ability to detect differences in protein or metabolite levels within several subcellular compartments. To overcome this hurdle, attempts have focused on subcellular fractionation of samples before analysis by using differential centrifugation (Drissi et al. 2013). Chen et al. also reported an immunoprecipitation method for isolation of intact mitochondria rapidly for metabolomics analyses (Chen et al. 2016a). As instrument sensitivity continues to improve, single-cell and subcellular analyses will become more feasible.
Nevertheless, advances in innovative omics technologies have contributed to success in generating the redox transcriptome, proteome, and metabolome as well as rapid accumulation of large-scale data. The global study of redox biology needs to combine these heterogeneous or broad omics data to better understand the mechanism of redox metabolism and its consequences for human diseases (Wang et al. 2018a). The limitation of metabolomic techniques is that they do not recognize directly ROS and NO; however, certain molecules such as succinate, oxidized glutathione, and urea cycle intermediates involved in RN reactions can be measured and analyzed, thus allowing an indirect examination of these pathways (Deidda et al. 2018).
Besides, information about identified metabolites’ biochemical pathways and biological function, both in physiological and pathological conditions, is essential for the proper understanding of the findings resulting from metabolomics application to the investigation of ROS and NO metabolism and signaling (Deidda et al. 2018).
Management of CVDs
For CVDs prevention and treatment, a healthy lifestyle is highly recommended because it is safe and cheap (Fyhrquist et al. 2013). This lifestyle includes ideal calorie intake, both good nutrition and psychological status as well as avoiding smoking (Fontana 2018).
Nutritional modulation by calorie restriction for the management of obesity and CVD
Calorie restriction (CR) has useful impacts on cardiovascular health by mechanisms including suppression of chronic inflammation and dyslipidemia as well as stimulation of immune response caused by increased cytokine, adipokine production (Meydani et al. 2016). Sirtuins a family of nicotinamide adenine dinucleotide (NAD)-dependent histone deacetylases (HDACs) (Singh et al. 2017) mainly Sirt1 controls various aspects of the caloric restriction (CR) response including insulin secretion, glucose homeostasis, fat metabolism, physical activity, and stress resistance (Guarente 2013). Stimulation of Sirt1 confers anti-oxidative and anti-inflammatory effects in the vasculature, causing attenuated vascular senescence (Kida and Goligorsky 2016). Besides, Sirt1 plays a significant role in the cardiac adaptive response to various stresses, such as oxidative stress, I/R, and starvation (Hsu et al. 2010; Akkafa et al. 2015). Besides, cardiac Sirt1 mediates the cardioprotective effect of CR by suppressing local complement system activation after I/R (Yamamoto et al. 2016). CR primes cardiac mitochondria into a stress-resistant state, leading to improvements in myocardial ischemic tolerance. Based on the finding that enhanced sirtuin activity was associated with a decline in the amount of acetylated mitochondrial proteins in the CR heart, in this regard, the beneficial effect of CR on mitochondrial function is mediated by deacetylating specific mitochondrial proteins. Among numerous CR-induced deacetylated proteins, for example, the deacetylation of NDUFS1 (complex I) and/or Rieske subunit of cytochrome bc1 complex (complex III) plays a key role in the reduced ROS production in the mitochondria during early reperfusion. Low-dose resveratrol (RSV), a natural bioactive polyphenolic compound present in a wide variety of foods, particularly red grapes (Lee et al. 2014) also caused a lower ROS development and improved cell survival in cultivated neonatal cardiomyocytes following hypoxia/reoxygenation without raising expression levels of MnSOD in a sirtuin-based way (Shinmura et al. 2011). Additionally, CR inhibits insulin resistance (Ruggenenti et al. 2017); decreased oxidative damage for protein, lipid, and DNA leading to boosting endothelial function (Il’yasova et al. 2018).
A poor diet, rich in refined and preserved food as well as an imbalance of sleep and exercise, has resulted in an epidemic of obesity in the sedentary lifestyle. Individuals with a 30 or higher index of body mass are overweight and obese (Most et al. 2017). Obesity is a risk factor for many CVDs such as MI, hypertension, and atherosclerosis (Halade and Kain 2017). Obesity induces cardiovascular disease via ectopic lipid deposition, an increase of blood glucose level, and the development of a procoagulant state (Flegal et al. 2010). Obesity has been found to induce pro-inflammatory adipokines expression and diminished anti-inflammatory adipokines expressions such as TNFα and IL-6, developing a chronic, low-grade inflammatory condition (Flegal et al. 2012). Also, under obesity conditions, adipokines were shown to stimulate both metabolic diseases and CVDs (Ouchi et al. 2011).
Also, metabolic disease has been linked strongly with low-level inflammation. For instance, an adipose mass increase in women suffering from obesity is associated with an elevation in pro-inflammatory marker CRP levels in the serum (Zeng et al. 2021). Conversely, weight loss decreased serum IL-6 and CRP and levels (Meghatria and Belhamiti 2021). These results strongly demonstrated that an obesity-linked inflammatory state is due to changes in the expression of adipocytes cytokines (Samaras et al. 2010). For example, in obesity and diabetes models, adipose tissue TNFα expression is induced (Nakamura et al. 2014) and in the case of weight loss in obese people, there is a decline in the levels of TNFα (Ouchi et al. 2011).
Dietary/supplemental antioxidant and drugs with antioxidant/anti-inflammatory properties
As described before, there is a balance between the formation of ROS / free radical and endogenous antioxidant defense mechanisms in normal and healthy body conditions. If this balance is disrupted, it can lead to oxidative stress and damage to all essential cellular components such as DNA, proteins, and membrane lipids leading to cell death (Senoner and Dichtl 2019). Consequently, it can cause numerous diseases such as CVDs and inflammation (Arulselvan et al. 2016).
The cardioprotective effects could include antioxidation, anti-inflammation, antiplatelet, blood pressure reduction, lipid metabolism modification, blood glucose regulation, endothelial function improvement, and myocardial damage mitigation. Besides, the mechanisms of action could include the modulation of related enzyme activity, gene expression, and signaling pathways, as well as some other CVD-related biomarkers (Tang et al. 2017).
Phenolics and flavonoids contents are contributed to the antioxidant activity of natural ingredients. Also, trace metals such as Cu, Zn, Mg, Mn, and Se have been reported to perform significant functions in the antioxidant system (Ravipati et al. 2012). The use of naturally occurring antioxidants (Fig. 9) and the development of chemical antioxidants to reduce or prevent CVD are of importance over time, given the prevalence of CVD and the role of ROS in many cardiovascular pathologies, as stated above.
Phenolics and flavonoids have been considered anti-inflammatory compounds in addition to their antioxidant activity. The results of anti-inflammatory research for natural extracts and their active ingredients compounds proved by hindering both MAPK and NF- κB pathways, which function to produce numerous mediators for proinflammation (Arulselvan et al. 2016). Polyphenols may be classified into natural, semi-synthetic, and synthetic compounds, and have been known to minimize the risk of MI, stroke, and cardiovascular death (Peterson et al. 2012).
Studies have demonstrated that the intake of polyphenols can prevent CVD onset due to their antioxidant and antiatherosclerotic activity (Rizzi et al. 2016). It was found that grape polyphenols supplementation significantly decreased the serum TMAO level which is considered an emerging risk factor for CVD (Annunziata et al. 2019a, b). The individual response to polyphenols intake has been attributed to the PON1 enzyme gene which encodes for glycoprotein that is known to protect lipoproteins from oxidation and consequently strongly associated with HDL antioxidant and anti-inflammatory activity (Barrea et al. 2020).
Besides, RSV provides several benefits for health, including antioxidant activity, through increased antioxidant enzyme activities and free-radical scavenging (AlBasher et al. 2020). RSV also has many biological characteristics, such as anti-tumor, anti-inflammatory, anti-aging, anti-hyperlipidemic, cardioprotective as well as neuroprotective effects (Benayahoum et al. 2013). It was found that RSV stimulates a dose-dependent elevation in the activity of the NAD+ synthetic enzyme nicotinamide mononucleotide adenylyltransferase (NMNAT1) leading to increased NAD+ levels enhancing SIRT1 activity which used NAD+ as a substrate to induce its gene-silencing activity (Grant 2010). SIRT1 has been shown to deacetylate and activate eNOS (Guo et al. 2020), and after arterial injury, whole body or smooth muscle cell-specific overexpression of SIRT1 protected mice against neointimal hyperplasia (Li et al. 2011; Bae et al. 2013).
Extra virgin olive oil (EVOO) is rich in polyphenols. When comparing EVOO foods with corn oil meals, healthy individuals with EVOO intake show a considerable decrease in oxidative stress levels and markers of endothelial dysfunction because of the high polyphenolic contents of olive oil (Carnevale et al. 2014). Also, dark chocolate suppresses platelet function only in smokers by reducing oxidative stress; this effect appears to depend on its polyphenolic content (Carnevale et al. 2012). The distinct composition of nuts, which includes complex carbohydrates, unsaturated fats, protein, vitamins or fiber, minerals except for sodium, plant sterols, and other bioactive components, as polyphenols, makes nuts special among plant foods (Ros 2010). A reduced CVDs risk, as well as obesity, cancer, and diabetes mellitus, was associated with regular intake of nuts (Aune et al. 2016).
Besides, tea polyphenols, also known as catechins, were found to increase SOD and CAT activities, stimulate the free radicals scavenging, and decreased lipid peroxide formation. Also, they are involved in inducing apoptosis of VSMCs by upregulation of p21, p53, and NF-κB, thus decreasing the risk of atherosclerosis development (Chen et al. 2016b). In a systematic review to analyze the flavonoid’s impact on vascular function (Kay et al. 2012), it was found that they improved blood pressure and both acute and chronic flow-mediated dilation (FMD). Anthocyanins, a type of flavonoid with significant antioxidant effects and positively affect the vascular endothelium function (Heiss et al. 2010). Anthocyanin supplementation improved both acute and chronic FMD are improved with no improvement in the reactive hyperemia index.
It is well evident that both anthocyanins and polyphenols can prevent the onset of CVD (Du et al. 2016), due to their antiatherosclerotic and antioxidant and effects (Wallace et al. 2016). There is a very low bioavailability for phenolic compounds where only 10% of them are absorbed in the small intestine, whereas about 90% is excreted or metabolized by the intestinal microbiota (Kawabata et al. 2019). Therefore, the primary protective effect of anthocyanins cannot be due to the antioxidant properties, which will be active only at the intestinal level, but to their action as secondary intracellular mediators in different signaling pathways. Other studies highlighted the cardioprotective and anti-inflammatory effects of anthocyanins showed also anti-inflammatory and cardioprotective effects. In this regard, the intake of anthocyanins promotes NO production that improves blood circulation and, on the other hand, can inhibit Nf -kB transcription, reducing pro-inflammatory molecule production (Kruger et al. 2014). Also, in a randomized controlled clinical trial (RCT) to study the anti-inflammatory impact of anthocyanins (Zhu et al. 2013), a total of 150 subjects with hypercholesterolemia consumed a purified anthocyanins mixture (320 mg/day) or a placebo twice a day for 24 weeks. The intake of anthocyanins significantly diminished the levels of plasma IL-1β, serum CRP, soluble vascular cell adhesion molecule-1 compared with the placebo as well as a significant difference in the HDL and LDL-cholesterol levels between the two groups (Zhu et al. 2013).
Quercetin, a dietary antioxidant flavonoid, has a therapeutic and protective impact on lipopolysaccharide (LPS)-induced oxidative stress and vascular dysfunction in mice study (Kukongviriyapan et al. 2012). Quercetin improved the eNOS expression in the aortic tissues and reduced the levels of nitrate/nitrite while abolished the iNOS expression compared to the LPS injected mice. Also, it suppressed LPS increased aortic O2•− production and markedly reduced protein oxidation and lipid peroxidation as well as restored the GSH redox ratio. Moreover, in a hypercholesterolemia animal model study, quercetin showed a significant decrease in hepatic thiobarbituric acid reactive substances (TBARS) level and plasma levels of total lipids, whereas it markedly increased liver CAT activity and total thiol level as compared with control rats (Khamis et al. 2017).
In a previous study of our research group, it was found that hesperidin, a citrus fruit bioflavonoid, can protect against cardiomyopathy caused by doxorubicin via suppressing oxidative stress, inflammation, and apoptosis (Donia et al. 2018). The findings of a prospective study to assess the relationship between flavonoid consumption and cardiovascular death (McCullough et al. 2012), it was showed that study participants with the highest quintile total flavonoid intakes lowered the cardiovascular risk by 18%, regardless of the adjustment of many cardiovascular risk factors.
Oxidative stress is inversely correlated with the Mediterranean diet adherence, primarily because of the decrease of GSSG concentrations, even following to modification of different risk factors for CV (Duda-Chodak et al. 2015). Omega-3 or omega-6 polyunsaturated fatty acids have been found to minimize the occurrence of CVDs due to their anti-atherogenic, anti-thrombotic effects as well as their lowering effects on blood pressure (Sokoła-Wysoczańska et al. 2018). Eicosapentaenoic acid and docosahexaenoic acid, two n-3 polyunsaturated fatty acids, reduced inflammation, and boost endothelial function, thus inhibiting atherosclerosis development (Bhatt et al. 2019). They diminished endothelial DNA damage by ROS (Sakai et al. 2017). Also, their modified lipid effectors, namely resolvins, maresins, and protectins, have anti-inflammatory effects (Sies et al. 2017).
It was found that vitamin C is promising in studies with 10–55 participants to evaluate the impact of vitamin C in CHF (Daiber et al. 2017). Also, higher vitamin E intake in women and men lowers the coronary disease risk (Fihn et al. 2012). Many studies showed that vitamins C, E, and other antioxidants can decrease CVD by trapping organic free radicals and inhibiting excited oxygen molecules to prevent tissue damage (Lobo et al. 2010). Antioxidants may be able to slow or prevent atherosclerotic plaque formation, likely, by inhibiting the oxidation of LDL-cholesterol (Nordestgaard and Varbo 2014). However, the results of studies concerning the role of vitamin C and E in human CVD prevention are still controversial. Vitamin C supplements, exceeding 700 mg/day, were significantly linked to 25% decreases in coronary heart disease risk in a pooled analysis of nine cohorts (Widmer et al. 2015).
In RCT including 4641 middle-aged men to assess whether the long-term supplementation of vitamin E or vitamin C can decrease the risk of major cardiovascular events, it was indicated that either vitamin E or vitamin C cannot reduce the risk of major cardiovascular events by following 8 years (Zhu et al. 2021). Another RCT reported a possible anti-inflammatory effect of 500 mg of vitamin C twice a day in 64 people with obesity, hypertension, and/or diabetes. In this study, vitamin C might act by the induction of the decline in CRP, IL-6, and fasting blood glucose after 8 weeks of treatment might be induced by vitamin C (Ellulu et al. 2015).
Also, the intake of antioxidant vitamins (vitamin A, C, and E) from food and supplements in over 3000 postmenopausal women with no CVD was tested for a period of 7-years follow-up, indicating that the vitamin E intake from food was inversely associated with the death risk from coronary heart disease (Sugamura and Keaney Jr 2011).
The thiol-containing antioxidant N-acetylcysteine (NAC) can regenerate the intracellular antioxidant reservoirs, as NAC deacetylation produces cysteine, which is a precursor for GSH. Initial small trials evaluating the intravenous NAC efficacy on acute MI and ischemic heart disease were shown to be promising in infarct size limitation (Samuni et al. 2013).
Furthermore, probiotics use can enhance cardiovascular function and have beneficial effects on patients with heart failure (Vasquez et al. 2019). The regular intake of probiotics such as kefir which maintains the bowel balance could have partial cardiovascular benefits depending on reducing oxidative stress (Vasquez et al. 2019). Kefir has been found to protect from vascular dysfunction of the endothelium and incorrect impaired autonomic cardiovascular function (Silva-Cutini et al. 2019), including inhibition of the angiotensin-converting enzyme (Brasil et al. 2018). Probiotics may thus be a promising natural contributor to the prevention/therapy of CVD, including hypertension (Vasquez et al. 2019).
In a pilot study to address the probiotics prognostic effects in patients with heart failure class II or III and left ventricular ejection fraction, < 50% were randomized to probiotic therapy with Saccharomyces boulardii or placebo for 3 months in a double-blinded fashion (Costanza et al. 2015). The left atrial diameter, as well as the levels of CRP, UA, and creatinine, was reduced considerably in patients treated with probiotics. Therapy using probiotics was also safe and well-tolerated without reporting adverse consequences or negative events (Vasquez et al. 2019). More mechanistic studies are therefore required to identify the missing links to the safety of fermented foods, such as pre-, pro-and synbiotics, and of the bioactive compounds of fermented foods on blood pressure neural control (Vasquez et al. 2019).
Due to the limitation of the common antioxidants in attenuating heart disease and the strong animal models studies that promoted the concept that limiting mitochondrial ROS production or improved ROS mitochondrial scavenging can be extremely useful for the heart, thus there has been considerable effort to identify mitochondria-targeted antioxidants as drugs. MitoTEMPO, SS-31/MPT-131, and mitoquinone (mitoQ) have been reported a positive effect as mitochondria-targeted agents (Peoples et al. 2019). Mito TEMPO is a mitochondria-targeted agent mimicking SOD and composed by conjugating nitroxide of piperidine to the lipophilic triphenylphosphonium cation (TPP+), where the accumulation of it in the matrix depending on the membrane potential (Leopold 2015). It causes superoxide detoxification to restrict the formation of hydroxyl radicals via cycling between its oxoammonium and nitroxide forms, as well as ferrous iron oxidation (Zielonka et al. 2017). In an animal study, mitoTEMPO efficiently decreased the ROS production in both cytosolic and mitochondrial compartments in case of myocytes failure, and repeated administration could prevent heart failure (Dey et al. 2018).
The antioxidant MitoQ has been documented to reduce oxidative stress and increase the antioxidant GPX1, which catalyzes the conversion of peroxides into alcohol and water (Escribano-Lopez et al. 2016). Also, to modulate the leukocyte-endothelium interaction (Escribano-Lopez et al. 2016). The cardioprotective effect of MitoQ involves raising leucocyte velocity and reducing its flux and adhesion to the endothelium, thus limiting inflammation as well as oxidative stress (Escribano-Lopez et al. 2016). MitoQ is targeting ubiquinone attached to the TPP+ cation in the mitochondrial matrix. On targeting the matrix, ubiquinone is reduced to ubiquinol, which acts as a carrier for electrons to boost the complexes I / II electron transfer to complex III (Dryhurst 2012), and as well as it acts as an antioxidant by reducing the peroxidation of lipids (Victor et al. 2011).
In in vitro studies, mitoQ was found to significantly cause decreased oxidative damage and stimulated the protection against cell death which is induced H2O2 as well as chemically induced cardiac I/R injury (Bayeva et al. 2013). In in vivo IR injury Langendorff isolated heart model, it has been shown that mitoQ administration in drinking water to rats decreased cytochrome c release, mitochondrial damage, caspase 3 activations, and death of cardiomyocyte (Espinosa-Diez et al. 2015). This decrease in cardiomyocyte loss has been found to strongly link to high cardiac contractility and preserved mitochondrial function of the respiratory chain, so mitoQ has both mitoprotective and cardioprotective effects (Espinosa-Diez et al. 2015). It also maintained respiratory chain function and mitochondrial membrane potential as well as reduced the oxidant sensitivity which induced mitochondrial-permeability transition pore (Junior et al. 2018).
SS-31 which is D-Arg-2′6′-dimethylTyr-Lys-Phe-NH2 and its acetate salt MTP-131 (also called Elamipretide or Bendavia) are peptides with antioxidant activity, high membrane permeability, as well as solubility and, could target the mitochondria (Szeto 2014). Its accumulation at the inner mitochondrial membrane is independent of membrane potential, it could thus act on both healthy and diseased mitochondria. Specifically, SS-31 could bind to inner membrane phospholipid called cardiolipin, and regulates mtDNA nucleoid distribution, cristae architecture, respiratory chain complex integrity, and supercomplex organization (Rochette et al. 2014). Also, SS-31 modulates the interaction of cardiolipin with cytochrome-c specifically (Birk et al. 2013). The balance between the electron carrier and cytochrome-c peroxidase activities was shown to be regulated by cardiolipin to mediate oxidative mitochondrial damage. This balance is mediated through hydrophobic binding between cytochrome c and cardiolipin results in partial cytochrome c unfolding that boosts the activity of its peroxidase. SS-31 binds to cardiolipin and disrupting its interaction with cytochrome c, thus inducing the cytochrome c action as a respiratory chain electron carrier (Hanske et al. 2012).
Carvedilol functions as a mitochondrial complex-I inhibitor that is active in the cardiotoxicity-induced anthracycline mechanisms (Montezano and Touyz 2014). It also reduced the harmful effect of doxorubicin on the left ventricular ejection fraction and induced lipoperoxidation in vivo models (Carvalho et al. 2014).
Besides, the antioxidant composition may differ in patients, and before treatment, predicting antioxidant profiles can be useful in selecting the candidate for antioxidant therapy and avoiding over exposition. Also, monitoring of the antioxidant status of patients will allow the selection of subjects who may benefit from vitamin supplementation before and during therapy (Vardi et al. 2013). The correlation between patients’ vitamin lack and disease symptoms is suggested primarily by consuming oxidation-sensitive vitamins associated with inflammation, leading to a variety of secondary effects (Mangge et al. 2015).
CKD is associated with mitochondrial dysfunction. The common complication of CKD is iron deficiency anemia that is associated with poor clinical outcomes that affect the function of mitochondria and intensify oxidative stress (Nuhu et al. 2019). The increased risk of ROS development in iron deficiency anemia in CKD leading to increased RBC death, anemia, and oxidative stress severity (Rysz et al. 2020). It has been found that iron deficiency anemia, oxidative stress is partly mediated via antioxidant reduction (Prats et al. 2014) and timely replacement of iron (via intravenous route rather than oral) and antioxidant therapies could lead to clinical improvement. In this regard, iron therapy improved iron deficiency anemia in CKD without a significant impact on renal function or oxidant status (Nuhu et al. 2019).
In the treatment of patients with a proven atherosclerotic disease with highly specific anti-inflammatory therapies (for instance cytokines targeted monoclonal antibodies), there was a decreased cardiovascular mortality. Current antidiabetic cardiovascular drugs (e.g., sodium-glucose transport protein 2 (SGLT2) inhibitors, Dipeptidyl Peptidase-4 (DPP-4) inhibitors, and glucagon-like peptide-1(GLP-1) analogs) inhibit inflammatory and detrimental redox mechanisms and therefore decrease the CVDs risk (Steven et al. 2019).
Anti-inflammatory mechanisms for SGLT2 can include weight loss and reductions of ketone and UA as well as inflammation of the adipose tissue, or oxidation stress attenuation (Bonnet and Scheen 2018). It was found that therapy of obesity, hyperlipidemia, hyperglycemia, and oxidative stress, as well as various mice inflammation parameters with type 1 or 2 diabetes mellitus, were improved by SGLT2 inhibitor ipragliflozin (Tahara et al. 2014). Additionally, the SGLT2 inhibitor dapagliflozin ameliorated nephropathy and glucose homeostasis and decreased inflammation markers in the genetically diabetic model called db/db mice (Terami et al. 2014). Many SGLT2 inhibitors including luseogliflozin, empagliflozin, and dapagliflozin have anti-inflammatory effects in the progression of atherosclerosis (Han et al. 2017). Moreover, Shin et al. showed that dapagliflozin decreased oxidative stress by restoring Mn-SOD, Cu/Zn SOD, and CAT expression in renal tissues of diabetic animals (Shin et al. 2016).
In this regard, SGLT2 inhibitor TA-1887 inhibits oxidative damage by increasing CAT and Mn-SOD expression (Sugizaki et al. 2017). The empagliflozin has been reported to reduce free-radical production and improve oxidative stress-induced vascular dysfunction in the aortic vessels of diabetic rats via mitochondrial function recovery while suppressing pro-oxidant agent activity (Oelze et al. 2014). The cardiovascular safety profiles empagliflozin and canagliflozin were studied in the Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients-Removing Excess Glucose and Canagliflozin Cardiovascular Assessment Study trials, respectively (Rastogi and Bhansali 2017). Both studies indicated the use of SGLT2 inhibitors was beneficial and decreased cardiovascular deaths, as well as decreased incidence of MI and stroke (Neal et al. 2017). Given the antioxidant effects of the SGLT2 inhibitors detailed above, it is perhaps interesting to infer that this antioxidant effect may have contributed toward the reduction in cardiovascular events observed in the previously mentioned trials (Yaribeygi et al. 2019).
Statins have been found to significantly decrease the risk of key cardiovascular events (Collaboration 2012). They decreased the levels of proinflammatory chemokines and cytokines, adhesion molecules such as NF-κB and P-selectin, and monocyte activation. They also minimize oxidative stress via decreasing NOX and O2−. production as well as oxidation of LDL and by rising free radical scavenging. Moreover, statins increase eNOS activity and NO levels via increased BH4 bioavailability in patients with CAD (Antoniades et al. 2011).
Also, statins serve as inhibitors of 3-hydroxy-3 methylglutaryl- coenzyme A and suggested to minimize the risk of coronary heart disease due to their lipid reduction effects (Dagli et al. 2010). Colchicine has also been used as an adjuvant to atherosclerosis treatment based on its anti-inflammatory effects. Thereby decreasing the production of monocyte proinflammatory cytokines such as IL-1β and IL-18 as well as inhibiting the functioning and activation of neutrophils (Martínez et al. 2018).
To prevent cardiovascular events in CKD patients, ideally targeted intervention goals should include control of blood pressure, lipid, diabetes, proteinuria reduction, anemia treatment, management of metabolic abnormalities, and lifestyle changes including smoking cessation, low salt consumption, achieving a normal body mass index. The use of β blockers, renin-angiotensin blockers, diuretics, statins, and aspirin is helpful in the early stages of CKD (Liu et al. 2014).
Exercise training and hormesis as an adaptive response to oxidative stress
Physical activity and exercise, both aerobic and anaerobic, lead to the prevention of CVD (Kokkinos and Myers 2010). Consequently, maximal levels of exercise capacity are known to suppress cardiovascular mortality and morbidity. On the other hand, acute exercise stimulates the ROS overproduction leading to an imbalance between free radical production and the antioxidant defense causing oxidative stress (Dekleva et al. 2012). It was known that physical inactivity and decrease aerobic capacity are independent and potent for CVD mortality prediction. However, an increase in physical exercise levels cannot decline the higher mortality risk in case of extensive adiposity (Berrington de Gonzalez et al. 2010). Ideally, regular exercise practice has a significant impact on optimizing cardiovascular and metabolic health and can protect against obesity by elevating the biogenesis of mitochondria as well as the consumption of calories and oxygen (Holloszy 2011). Also, it upregulates glucose transporter type 4 as well as glycogen synthase leading to insulin responsiveness in muscle (Soufi et al. 2014). In the case of obesity-associated with hypertension, regular exercise upregulates the skeletal muscle lipoprotein lipase lead to an optimal lipid profile (Richter and Hargreaves 2013), and a decrease in blood pressure level (Bouchard et al. 2011).
Besides, the important role of endurance exercise to protect against endothelial dysfunction and arterial stiffening is mediated via its significant antioxidative stress and anti-inflammatory effects. For example, the wheel running in the animal study increases NO bioavailability, this may be due to an increase in shear stress which raises the AKT1 expression and subsequent phosphorylation eNOS. Consequently, it decreased the rate of accumulation of nitrotyrosine as well as the end products of advanced glycation, and the decrease in NOX expression in the vasculature as well as NF- κB leading to a decrease in serum cytokines concentration (Seals et al. 2014). Besides, in calorie restriction for induction of weight loss via anaerobic or isometric resistance training, fat loss and basal metabolic rate were found to be induced resulting in boosting cardiovascular health (Villareal et al. 2017). In patients with insulin resistance, it was recognized that the combination between anaerobic isometric exercise and regular exercise has better valuable impacts than regular exercise alone to increase insulin sensitivity and glucose tolerance while reducing body fat (Villareal et al. 2017).
In resting conditions, the complex antioxidant ROS protection mechanisms are often not enough to avoid oxidative stress during or after exercise. Anaerobic exercise is sometimes characterized as a short-term, physical activity with high intensity and often as intense exercise. All types of anaerobic activity enhance ROS production and cause oxidative stress. As oxygen usage is increased to a lesser extent during and after anaerobic exercise than in aerobic workouts (Nocella et al. 2019).
Activation of respiration in mitochondria is not the major endogenous cause to produce ROS. Instead, anaerobic exercise-induced increases in ROS are proposed to be derived primarily from the active generation of radicals by XO and NOX as well as from phagocytic respiratory burst and prostanoid metabolism, disruption of iron-containing proteins, and calcium homeostasis loss. For example, XO, a major contributory enzyme to ROS formation during exercise, is produced by ischemia-induced xanthine dehydrogenase proteolysis which takes place during strenuous exercise; its use of molecular oxygen as the electron acceptor results in the production of O2•- and H2O2 (Veskoukis et al. 2012).
Hormesis is defined by a dose-response connection in which low-dose stimulation results in several positive physiological responses, but high-dose stimulation has harmful consequences. Then, the hormesis concept has been generalized to “adaptive response”, in which living organisms chronically exposed to low-dose hazardous substances become resistant to subsequent high-dose exposure to the same stimulant (Reuter et al. 2010). In other words, repeated low-dose stimuli can cause different hormetic adaptive responses, including antioxidant activation, DNA repair function, and immune function. Also, it was thought that mild or moderate exercise-induced oxidative stress may cause hormetic adaptive reactions. Thus, physiological enhancement (in this case, oxidative stress caused by exercise) is necessary for obtaining health-promoting improvement (e.g., improved antioxidant defense) but levels must never go beyond the oxidative harm upper threshold (Calabrese et al. 2011).
Antioxidant supplementation might be advised before or after the stressful or competitive workout to reduce exercise-induced oxidative damage. Also, the rates of oxidant stress triggered by a high intensity or prolonged exercise may overburden the capacities of antioxidant protection (Koyama 2014). For instance, the practice of an acute exercise enhances the expression of SOD2 in the mitochondria of rat skeletal muscle results in an increase in its activity and inhibiting the accumulation of superoxide after practicing exercise (Steinbacher and Eckl 2015) and this could illustrate the hormesis adaptive principle. Also, a greater frequency of multiple diseases is caused by physical inactivity. Normal exercise, on the other hand, is moderately intense and lasting and has several advantageous effects on the body such as enhancing cardiovascular function, partially due to a nitric oxide-mediated adaptation (Radak et al. 2019).
Antioxidants in high concentrations may have pro-oxidant effects (Yang et al. 2018a) and excessive antioxidants can have dangerous consequences. For example, small but continuous production of ROS expression during physical exercise enhances antioxidant defenses and induces expression of antioxidant enzymes; The supplementation with vitamin C increases stamina in humans and rats (Di Meo et al. 2016) and decreases some of the improved skeletal muscle adaptations after acute exercise (Morrison et al. 2015). Although physiological antioxidant doses may be beneficial, excessive antioxidation may have deleterious implications because the “remodeling” of exercised skeletal muscles depends on reactive oxygen and nitrogen signaling (Merry and Ristow 2016). The duration of antioxidant effects can also be temporary. For instance, in pre-and stage1 hypertensive postmenopausal women, the biomarker of oxidant DNA damage was mitigated by regular consumption of blueberries for four weeks, but these effects were not discovered after 8 weeks (Johnson et al. 2017).
Mindfulness and stress control
Permanent psychological stress (such as work-related stress, marital stress, caring for a sick spouse, or chronic stress associated psychological condition) and negative emotions, independently of other risk factors, have adverse effects on cardiovascular health and increase the risk of arrhythmias (Kivimäki and Steptoe 2018). It was found that these psychosocial factors were the third most important risk factors for MI following smoking and high Apolipoprotein B/ apolipoprotein A- I. A meta-analysis indicates that the risk of low cardiovascular prognosis is almost doubled by depression after MI (Lichtman et al. 2014). The mechanism for this is by affecting the hypothalamic, pituitary-adrenal axis, and autonomous nervous system leading to an elevation in plasma stress hormones (for example, catecholamines and corticosteroid) causing the change in blood pressure and both immune and platelet response and finally stimulate the oxidative stress and inflammation (Carney and Freedland 2017).
In this regard, excessive endogenous production or increased exogenous catecholamine administration has been found to induce cardiotoxicity (Li et al. 2020). In this regard, the pathogenesis of cardiac damage has been reported to link to the accumulation of intracellular Ca2+ through induced oxidative mechanisms by catecholamines (Costa et al. 2011). By oxidation of catecholamines, unstable catecholamines-O-quinones are produced that in turn converted into the respective adrenochromes and subsequently oxygen-free radicals such as superoxide radicals are produced. By the action of SOD, the superoxide radicals can be reduced to hydrogen peroxide, which damages the membrane integrity. Superoxide radical was suggested to cause damage in cardiomyopathy caused by catecholamine (Pelliccia et al. 2017). In conjunction with the concomitant production of reactive intermediates and free radicals by enzymatic or metal-catalyzed auto-oxidation of catecholamine, this leads to their toxicities which affect particularly heart tissues (Yang et al. 2018b). Additionally, norepinephrine injection causing the cardiac dysfunction in jeopardized myocardium is due to the elevation of the cardioinhibitory cytokines which may be produced as an adaptive response (Neri et al. 2015).
Concerning the relationship between glucocorticoids and oxidative stress, a meta-analysis shows that, depending on the duration of the therapy, glucocorticoids have been substantially induced oxidative stress. Essentially, the heart was the least tissue vulnerable to glucocorticoids induced oxidative stress while the brain is the most vulnerable to this stress. However, in studies involving both genders, the impact size was greater (1) relative only to males, (2) when corticosterone was used instead of dexamethasone, and (3) in juveniles rather than adults (Costantini et al. 2011).
High cortisol levels have been associated with increased levels of oxidative damage (Joergensen et al. 2011). During mood episodes, this allostatic load causes damage which is hypothesized to make an individual more vulnerable to develop the following episode and somatic disease at higher risk (Grande et al. 2012). Besides, increased cortisol secretion with chronic inflammation of low-grade decreases eNOS expression and NO formation (Bernatova 2014). Depression increased the concentration of inflammatory markers and endothelial dysfunction (Huffman et al. 2013). Experimentally, persistent stress has been found to cause dramatic changes in heart function such as fibrous tissue accumulation in the left ventricular myocardium, left ventricular diastolic damage, and maladaptive cardiac hypertrophy. Also, there was elevated plaque formation in the coronary arteries in response to atherosclerosis, alteration in the heart electrical conduction system (including reduced myocardial refractoriness and diminished conduction), and raised cardiac arrhythmias risk (Crestani 2016).
Conclusion
Conclusively, throughout this review, we have presented evidence in the literature indicating that cardiovascular diseases as one of the leading reasons for global death and many research studies to discover the symptoms, risk factors, effective tools in diagnosis, cellular and molecular mechanisms as well as laboratory biomarkers and finally targeting cardiovascular disease with the effective therapy focusing on oxidative stress and inflammation (as described in Fig. 10). Also, this review covers the area of using miRNA and omics technologies in understanding the cellular and molecular mechanisms underlying several cardiovascular diseases with detecting and hope to overcome their challenges. Dyslipidemia, smoking, hypertension, diabetes mellitus, and chronic kidney disease are well-documented as risk factors for cardiovascular diseases which ultimately lead to oxidative stress and stimulate inflammation. Therefore, natural or synthetic antioxidants and anti-inflammatory agents are recommended to improve cardiovascular health but sometimes the antioxidants targeting the mitochondria are significant to mitigate cardiovascular disease. Besides, a healthy lifestyle includes ideal calorie intake and healthy food as well as good psychological status, with no smoking, is of significant positive impact to prevent or treat cardiovascular dysfunction. There is a need to study both molecular and cellular mechanisms of different compounds that showed therapeutic or preventive effects against CVD. Also, more research is needed to manage different risk factors during the treatment of CVD by the discovery of new diagnostic or prognostic makers using several clinical trials.
Data availability
Not applicable.
References
Akkafa F, Halil Altiparmak I, Erkus ME, Aksoy N, Kaya C, Ozer A, Sezen H, Oztuzcu S, Koyuncu I, Umurhan B (2015) Reduced SIRT1 expression correlates with enhanced oxidative stress in compensated and decompensated heart failure. Redox Biol 6:169–173. https://doi.org/10.1016/j.redox.2015.07.011
Al Ghouleh I, Khoo NK, Knaus UG, Griendling KK, Touyz RM, Thannickal VJ, Barchowsky A, Nauseef WM, Kelley EE, Bauer PM (2011) Oxidases and peroxidases in cardiovascular and lung disease: new concepts in reactive oxygen species signaling. Free Radic Biol Med 51:1271–1288. https://doi.org/10.1016/j.freeradbiomed.2011.06.011
AlBasher G, Abdel-Daim MM, Almeer R, Ibrahim KA, Hamza RZ, Bungau S, Aleya L (2020) Synergistic antioxidant effects of resveratrol and curcumin against fipronil-triggered oxidative damage in male albino rats. Environ Sci Pollut Res Int 27(6):6505–6514. https://doi.org/10.1007/s11356-019-07344-8
Amelio D, Garofalo F, Imbrogno S, Tota B (2015) The occurrence and function of the NOS/NO system in the heart of the eel and African lungfish Phylogeny, anatomy, and physiology of ancient fishes. CRC Press, Taylor & Francis Group, Boca Raton, pp 19–37
Annunziata G, Maisto M, Schisano C, Ciampaglia R, Narciso V, Hassan ST, Tenore GC, Novellino E (2019a) Effect of grape pomace polyphenols with or without pectin on TMAO serum levels assessed by LC/MS-based assay: A preliminary clinical study on overweight/obese subjects. Front Pharmacol 10:575. https://doi.org/10.3389/fphar.2019.00575
Annunziata G, Maisto M, Schisano C, Ciampaglia R, Narciso V, Tenore GC, Novellino E (2019b) Effects of grape pomace polyphenolic extract (Taurisolo®) in reducing TMAO serum levels in humans: preliminary results from a randomized, placebo-controlled, cross-over study. Nutrients 11:139. https://doi.org/10.3390/nu11010139
Antoniades C, Bakogiannis C, Leeson P, Guzik TJ, Zhang M, Tousoulis D, Antonopoulos AS, Demosthenous M, Marinou K, Hale A (2011) Rapid, direct effects of statin treatment on arterial redox state and nitric oxide bioavailability in human atherosclerosis via tetrahydrobiopterin-mediated endothelial nitric oxide synthase coupling. Circulation 124:335–345. https://doi.org/10.1161/circulationaha.110.985150
Arulselvan P, Fard M, Tan W, Gothai S, Fakurazi S, Norhaizan M, Kumar S (2016) Role of antioxidants and natural products in inflammation. Oxidative Med Cell Longev 2016:5276130. https://doi.org/10.1155/2016/5276130
Audrain-McGovern J, Benowitz N (2011) Cigarette smoking, nicotine, and body weight. Clin Pharmacol Ther 90:164–168. https://doi.org/10.1038/clpt.2011.105
Aune D, Keum N, Giovannucci E, Fadnes L, Boffetta P, Greenwood DC, Tonstad S, Vatten L, Riboli E, Norat T (2016) Nut consumption and risk of cardiovascular disease, total cancer, all-cause and cause-specific mortality: a systematic review and dose-response meta-analysis of prospective studies. BMC Med 14:207. https://doi.org/10.1186/s12916-016-0730-3
Bae JU, Lee SJ, Seo KW, Kim YH, Park SY, Bae SS, Kim CD (2013) SIRT1 attenuates neointima formation by inhibiting HIF-1α expression in neointimal lesion of a murine wire-injured femoral artery. Int J Cardiol 168(4):4393–4396. https://doi.org/10.1016/j.ijcard.2013.05.044
Bai Y, Cui W, Xin Y, Miao X, Barati MT, Zhang C, Chen Q, Tan Y, Cui T, Zheng Y (2013) Prevention by sulforaphane of diabetic cardiomyopathy is associated with up-regulation of Nrf2 expression and transcription activation. J Mol Cell Cardiol 57:82–95. https://doi.org/10.1016/j.yjmcc.2013.01.008
Banerjee J, Khanna S, Bhattacharya A (2017) MicroRNA regulation of oxidative stress. Hindawi. https://doi.org/10.1155/2017/2872156
Barrea L, Annunziata G, Bordoni L, Muscogiuri G, Colao A, Savastano S (2020) Nutrigenetics—personalized nutrition in obesity and cardiovascular diseases. Int J Obes Suppl 10:1–13. https://doi.org/10.1038/s41367-020-0014-4
Bayeva M, Gheorghiade M, Ardehali H (2013) Mitochondria as a therapeutic target in heart failure. J Am Coll Cardiol 61:599–610. https://doi.org/10.1016/j.jacc.2012.08.1021
Benayahoum A, Amira-Guebailia H, Houache O (2013) A DFT method for the study of the antioxidant action mechanism of resveratrol derivatives. J Mol Model 19(6):2285–2298. https://doi.org/10.1007/s00894-013-1770-7
Bernatova I (2014) Endothelial dysfunction in experimental models of arterial hypertension: cause or consequence? Biomed Res Int 2014. https://doi.org/10.1155/2014/598271
Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, Moore SC, Tobias GS, Anton-Culver H, Freeman LB (2010) Body-mass index and mortality among 1.46 million white adults. N Engl J Med 363:2211–2219. https://doi.org/10.1056/NEJMoa1000367
Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT Jr, Juliano RA, Jiao L, Granowitz C (2019) Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 380:11–22. https://doi.org/10.1056/NEJMoa1812792
Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE (2014) Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev 94:329–354. https://doi.org/10.1152/physrev.00040.2012
Birk AV, Liu S, Soong Y, Mills W, Singh P, Warren JD, Seshan SV, Pardee JD, Szeto HH (2013) The mitochondrial-targeted compound SS-31 re-energizes ischemic mitochondria by interacting with cardiolipin. J Am Soc Nephrol 24:1250–1261. https://doi.org/10.1681/ASN.2012121216
Bonetta R (2018) Potential therapeutic applications of MnSODs and SOD-mimetics. Chem Eur J 24:5032–5041. https://doi.org/10.1002/chem.201704561
Bonnet F, Scheen A (2018) Effects of SGLT2 inhibitors on systemic and tissue low-grade inflammation: the potential contribution to diabetes complications and cardiovascular disease. Diabetes Metab 44:457–464. https://doi.org/10.1016/j.diabet.2018.09.005
Bonora M, Wieckowski MR, Chinopoulos C, Kepp O, Kroemer G, Galluzzi L, Pinton P (2015) Molecular mechanisms of cell death: central implication of ATP synthase in mitochondrial permeability transition. Oncogene 34:1475–1486. https://doi.org/10.1038/onc.2014.96
Bonora M, Wieckowski MR, Sinclair DA, Kroemer G, Pinton P, Galluzzi L (2019) Targeting mitochondria for cardiovascular disorders: therapeutic potential and obstacles. Nat Rev Cardiol 16:33–55. https://doi.org/10.1038/s41569-018-0074-0
Bouayed J, Bohn T (2010) Exogenous antioxidants—double-edged swords in cellular redox state: health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxidative Med Cell Longev 3:228–237. https://doi.org/10.4161/oxim.3.4.12858
Bouchard C, Sarzynski MA, Rice TK, Kraus WE, Church TS, Sung YJ, Rao DC, Rankinen T (2011) Genomic predictors of the maximal O2 uptake response to standardized exercise training programs. J Appl Physiol. https://doi.org/10.1152/japplphysiol.00973.2010
Brandes RP, Weissmann N, Schröder K (2014) Nox family NADPH oxidases: molecular mechanisms of activation. Free Radic Biol Med 76:208–226. https://doi.org/10.1016/j.freeradbiomed.2014.07.046
Brasil GA, de Almeida S-CM, Moraes F, Pereira T, Vasquez EC, Lenz D, Bissoli NS, Endringer DC, de Lima EM, Biancardi VC (2018) The benefits of soluble non-bacterial fraction of kefir on blood pressure and cardiac hypertrophy in hypertensive rats are mediated by an increase in baroreflex sensitivity and decrease in angiotensin-converting enzyme activity. Nutrition 51:66–72. https://doi.org/10.1016/j.nut.2017.12.007
Brigelius-Flohé R, Flohé L (2011) Basic principles and emerging concepts in the redox control of transcription factors. Antioxid Redox Signal 15:2335–2381. https://doi.org/10.1089/ars.2010.3534
Brito R, Castillo G, Gonzalez J, Valls N, Rodrigo R (2015) Oxidative stress in hypertension: mechanisms and therapeutic opportunities. Exp Clin Endocrinol Diabetes 123:325–335. https://doi.org/10.1055/s-0035-1548765
Brooks SD, Hileman SM, Chantler PD, Milde SA, Lemaster KA, Frisbee SJ, Shoemaker JK, Jackson DN, Frisbee JC (2018) Protection from vascular dysfunction in female rats with chronic stress and depressive symptoms. Am J Physiol Heart Circ Physiol 314:H1070–H1084. https://doi.org/10.1152/ajpheart.00647.2017
Buettner GR, Wagner BA, Rodgers VG (2013) Quantitative redox biology: an approach to understand the role of reactive species in defining the cellular redox environment. Cell Biochem Biophys 67:477–483. https://doi.org/10.1007/s12013-011-9320-3
Calabrese EJ, Stanek EJ III, Nascarella MA (2011) Evidence for hormesis in mutagenicity dose–response relationships. Mutat Res Genet Toxicol Environ Mutagen 726:91–97. https://doi.org/10.1016/j.mrgentox.2011.04.006
Carnevale R, Loffredo L, Pignatelli P, Nocella C, Bartimoccia S, Di Santo S, Martino F, Catasca E, Perri L, Violi F (2012) Dark chocolate inhibits platelet isoprostanes via NOX2 down-regulation in smokers. J Thromb Haemost 10:125–132. https://doi.org/10.1111/j.1538-7836.2011.04558.x
Carnevale R, Pignatelli P, Nocella C, Loffredo L, Pastori D, Vicario T, Petruccioli A, Bartimoccia S, Violi F (2014) Extra virgin olive oil blunt post-prandial oxidative stress via NOX2 down-regulation. Atherosclerosis 235:649–658. https://doi.org/10.1016/j.atherosclerosis.2014.05.954
Carney RM, Freedland KE (2017) Depression and coronary heart disease. Nat Rev Cardiol 14:145. https://doi.org/10.1038/nrcardio.2016.181
Carvalho FS, Burgeiro A, Garcia R, Moreno AJ, Carvalho RA, Oliveira PJ (2014) Doxorubicin-induced cardiotoxicity: from bioenergetic failure and cell death to cardiomyopathy. Med Res Rev 34:106–135. https://doi.org/10.1002/med.21280
Chen Q, Jin M, Yang F, Zhu J, Xiao Q, Zhang L (2013) Matrix metalloproteinases: inflammatory regulators of cell behaviors in vascular formation and remodeling. Mediat Inflamm. https://doi.org/10.1155/2013/928315
Chen WW, Freinkman E, Wang T, Birsoy K, Sabatini DM (2016a) Absolute quantification of matrix metabolites reveals the dynamics of mitochondrial metabolism. Cell 166:1324–1337. e1311. https://doi.org/10.1016/j.cell.2016.07.040
Chen X-Q, Hu T, Han Y, Huang W, Yuan H, Zhang Y, Du Y, Jiang Y (2016b) Preventive effects of catechins on cardiovascular disease. Molecules 21:1759. https://doi.org/10.3390/molecules21121759
Chen X, Qi L, Fan X, Tao H, Zhang M, Gao Q, Liu Y, Xu T, Zhang P, Su H (2019) Prenatal hypoxia affected endothelium-dependent vasodilation in mesenteric arteries of aged offspring via increased oxidative stress. Hypertens Res 42:863–875. https://doi.org/10.1038/s41440-018-0181-7
Choi S, Liu X, Pan Z (2018) Zinc deficiency and cellular oxidative stress: prognostic implications in cardiovascular diseases. Acta Pharmacol Sin 39:1120–1132. https://doi.org/10.1038/aps.2018.25
Chorley BN, Campbell MR, Wang X, Karaca M, Sambandan D, Bangura F, Xue P, Pi J, Kleeberger SR, Bell DA (2012) Identification of novel NRF2-regulated genes by ChIP-Seq: influence on retinoid X receptor alpha. Nucleic Acids Res 40:7416–7429. https://doi.org/10.1093/nar/gks409
Clapier CR, Iwasa J, Cairns BR, Peterson CL (2017) Mechanisms of action and regulation of ATP-dependent chromatin-remodelling complexes. Nat Rev Mol Cell Biol 18:407–422. https://doi.org/10.1038/nrm.2017.26
Clevenger LA (2016) The influence of early life adversity and recent life stress on psychological trajectories in women with ovarian cancer. https://doi.org/10.17077/etd.u4opbw7b
Coats A, Jain S (2017) Protective effects of nebivolol from oxidative stress to prevent hypertension-related target organ damage. J Hum Hypertens 31:376–381. https://doi.org/10.1038/jhh.2017.8
Collaboration ERF (2012) C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med 367:1310–1320. https://doi.org/10.1056/NEJMoa1107477
Colpaert RM, Calore M (2019) microRNAs in cardiac diseases. Cells 8:737. https://doi.org/10.3390/cells8070737
Costa VM, Carvalho F, Bastos ML, Carvalho RA, Carvalho M, Remião F (2011) Contribution of catecholamine reactive intermediates and oxidative stress to the pathologic features of heart diseases. Curr Med Chem 18(15):2272–2314. https://doi.org/10.2174/092986711795656081
Costantini D, Marasco V, Møller AP (2011) A meta-analysis of glucocorticoids as modulators of oxidative stress in vertebrates. J Comp Physiol B 181:447–456. https://doi.org/10.1007/s00360-011-0566-2
Costanza AC, Moscavitch SD, Neto HCF, Mesquita ET (2015) Probiotic therapy with Saccharomyces boulardii for heart failure patients: a randomized, double-blind, placebo-controlled pilot trial. Int J Cardiol 179:348–350. https://doi.org/10.1016/j.ijcard.2014.11.034
Craige SM, Kant S, Keaney JF Jr (2015) Reactive oxygen species in endothelial function–from disease to adaptation. Circ J 79:1145–1155. https://doi.org/10.1253/circj.CJ-15-0464
Crestani CC (2016) Emotional stress and cardiovascular complications in animal models: a review of the influence of stress type. Front Physiol 7:251. https://doi.org/10.3389/fphys.2016.00251
Dagli N, Poyrazoglu OK, Ferda Dagli A, Sahbaz F, Karaca I, Ali Kobat M, Bahcecioglu IH (2010) Is inflammatory bowel disease a risk factor for early atherosclerosis? Angiology 61:198–204. https://doi.org/10.1177/0003319709333869
Daiber A, Steven S, Weber A, Shuvaev VL, Muzykantov V, Laher I, Li H, Lamas S, Münzel T (2017) Targeting vascular (endothelial) dysfunction. Br J Pharmacol 174:1591–1619. https://doi.org/10.1111/bph.13517
Daugherty A, Daugherty A, Tall AR, Daemen M, Falk E, Fisher EA, García-Cardeña G, Lusis AJ, Owens A III, Rosenfeld ME, Virmani R (2017) Recommendation on design, execution, and reporting of animal atherosclerosis studies: a scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol 37:e131–e157. https://doi.org/10.1161/ATV.0000000000000062
Davies SS, Roberts LJ II (2011) F2-isoprostanes as an indicator and risk factor for coronary heart disease. Free Radic Biol Med 50:559–566. https://doi.org/10.1016/j.freeradbiomed.2010.11.023
Deidda M, Noto A, Bassareo PP, Cadeddu Dessalvi C, Mercuro G (2018) Metabolomic approach to redox and nitrosative reactions in cardiovascular diseases. Front Physiol 9:672. https://doi.org/10.3389/fphys.2018.00672
Dekleva M, Lazic JS, Pavlovic-Kleut M, Mazic S, Stevanovic A, Soldatovic I, Markovic-Nikolic N, Beleslin B (2012) Cardiopulmonary exercise testing and its relation to oxidative stress in patients with hypertension. Hypertens Res 35:1145–1151. https://doi.org/10.1038/hr.2012.115
Delbridge LM, Mellor KM, Taylor DJ, Gottlieb RA (2017) Myocardial stress and autophagy: mechanisms and potential therapies. Nat Rev Cardiol 14:412. https://doi.org/10.1038/nrcardio.2017.35
Dey S, DeMazumder D, Sidor A, Foster DB, O’Rourke B (2018) Mitochondrial ROS drive sudden cardiac death and chronic proteome remodeling in heart failure. Circ Res 123:356–371. https://doi.org/10.1161/CIRCRESAHA.118.312708
Di Meo S, Reed TT, Venditti P, Victor VM (2016) Role of ROS and RNS sources in physiological and pathological conditions. Oxidative Med Cell Longev. https://doi.org/10.1155/2016/1245049
Dikalov SI, Ungvari Z (2013) Role of mitochondrial oxidative stress in hypertension. Am J Physiol Heart Circ Physiol 305:H1417–H1427. https://doi.org/10.1152/ajpheart.00089.2013
Dong Y, Xu W, Liu C, Liu P, Li P, Wang K (2019) Reactive oxygen species related noncoding RNAs as regulators of cardiovascular diseases. Int J Biol Sci 15:680. https://doi.org/10.7150/ijbs.30464
Donia T, Eldaly S, Ali EM (2018) Ameliorating oxidative stress and inflammation by Hesperidin and vitamin E in doxorubicin induced cardiomyopathy. Turk J Biochem 44:207–217. https://doi.org/10.1515/tjb-2018-0156
D'Onofrio N, Servillo L, Balestrieri ML (2018) SIRT1 and SIRT6 signaling pathways in cardiovascular disease protection. Antioxid Redox Signal 28:711–732. https://doi.org/10.1089/ars.2017.7178
Drissi R, Dubois ML, Boisvert FM (2013) Proteomics methods for subcellular proteome analysis. FEBS J 280:5626–5634. https://doi.org/10.1111/febs.12502
Dryhurst G (2012) Biological electrochemistry vol 1. Elsevier
Du G, Sun L, Zhao R, Du L, Song J, Zhang L, He G, Zhang Y, Zhang J (2016) Polyphenols: Potential source of drugs for the treatment of ischaemic heart disease. Pharmacol Ther 162:23–34. https://doi.org/10.1016/j.pharmthera.2016.04.008
Duda-Chodak A, Tarko T, Satora P, Sroka P (2015) Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: a review. Eur J Nutr 54(3):325–341. https://doi.org/10.1007/s00394-015-0852-y
Duthie SJ (2011) Epigenetic modifications and human pathologies: cancer and CVD. Proc Nutr Soc 70(1):47–56. https://doi.org/10.1017/S0029665110003952
Ellulu MS, Rahmat A, Patimah I, Khaza'ai H, Abed Y (2015) Effect of vitamin C on inflammation and metabolic markers in hypertensive and/or diabetic obese adults: a randomized controlled trial. Drug Des Dev Ther 9:3405–3412. https://doi.org/10.2147/DDDT.S83144
Escribano-Lopez I, Diaz-Morales N, Rovira-Llopis S, de Marañon AM, Orden S, Alvarez A, Bañuls C, Rocha M, Murphy M, Hernandez-Mijares A (2016) The mitochondria-targeted antioxidant MitoQ modulates oxidative stress, inflammation and leukocyte-endothelium interactions in leukocytes isolated from type 2 diabetic patients. Redox Biol 10:200–205. https://doi.org/10.1016/j.redox.2016.10.017
Espinosa-Diez C, Miguel V, Mennerich D, Kietzmann T, Sánchez-Pérez P, Cadenas S, Lamas S (2015) Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol 6:183–197. https://doi.org/10.1016/j.redox.2015.07.008
Farah C, Michel LYM, Balligand JL (2018) Nitric oxide signalling in cardiovascular health and disease. Nat Rev Cardiol 15(5):292–316. https://doi.org/10.1038/nrcardio.2017.224
Fatehi-Hassanabad Z, Chan CB, Furman BL (2010) Reactive oxygen species and endothelial function in diabetes. Eur J Pharmacol 636(1-3):8–17. https://doi.org/10.1016/j.ejphar.2010.03.04
Ferretti G, Bacchetti T, Sahebkar A (2015) Effect of statin therapy on paraoxonase-1 status: a systematic review and meta-analysis of 25 clinical trials. Prog Lipid Res 60:50–73. https://doi.org/10.1016/j.plipres.2015.08.003
Fiedler J, Stöhr A, Gupta SK, Hartmann D, Holzmann A, Just A, Hansen A, Hilfiker-Kleiner D, Eschenhagen T, Thum T (2014) Functional microRNA library screening identifies the hypoxamir miR-24 as a potent regulator of smooth muscle cell proliferation and vascularization. Antioxid Redox Signal 21(8):1167–1176. https://doi.org/10.1089/ars.2013.5418
Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL, King SB 3rd, Kligfield PD, Krumholz HM, Kwong RY, Lim MJ, Linderbaum JA, Mack MJ, Munger MA, Prager RL, Sabik JF, Shaw LJ, Sikkema JD, Smith CR Jr, Smith SC Jr, Spertus JA, Williams SV, American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; American College of Physicians; American Association for Thoracic Surgery; Preventive Cardiovascular Nurses Association; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons (2012) ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 60(24):e44–e164. https://doi.org/10.1016/j.jacc.2012.07.013
Flegal KM, Carroll MD, Ogden CL, Curtin LR (2010) Prevalence and trends in obesity among US adults, 1999-2008. JAMA 303(3):235–241. https://doi.org/10.1001/jama.2009.2014
Flegal KM, Carroll MD, Kit BK, Ogden CL (2012) Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA 307(5):491–497. https://doi.org/10.1001/jama.2012.39
Foley RN, Curtis BM, Randell EW, Parfrey PS (2010) Left ventricular hypertrophy in new hemodialysis patients without symptomatic cardiac disease. Clin J Am Soc Nephrol 5(5):805–813. https://doi.org/10.2215/CJN.07761109
Fontana L (2018) Interventions to promote cardiometabolic health and slow cardiovascular ageing. Nat Rev Cardiol 15(9):566–577. https://doi.org/10.1038/s41569-018-0026-8
Förstermann U, Sessa WC (2012) Nitric oxide synthases: regulation and function. Eur Heart J 33(7):829–837, 837a-837d. https://doi.org/10.1093/eurheartj/ehr304
Förstermann U, Xia N, Li H (2017) Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ Res 120(4):713–735. https://doi.org/10.1161/CIRCRESAHA.116.309326
Furey TS (2012) ChIP-seq and beyond: new and improved methodologies to detect and characterize protein-DNA interactions. Nat Rev Genet 13(12):840–852. https://doi.org/10.1038/nrg3306
Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I (2017) Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 114:1752–1761. https://doi.org/10.1172/JCI21625
Fuster V, Lois F, Franco M (2010) Early identification of atherosclerotic disease by noninvasive imaging. Nat Rev Cardiol 7(6):327–333. https://doi.org/10.1038/nrcardio.2010.54
Fyhrquist F, Saijonmaa O, Strandberg T (2013) The roles of senescence and telomere shortening in cardiovascular disease. Nat Rev Cardiol 10(5):274–283. https://doi.org/10.1038/nrcardio.2013.30
Gawron-Skarbek A, Chrzczanowicz J, Kostka J, Nowak D, Drygas W, Jegier A, Kostka T (2014) Cardiovascular risk factors and total serum antioxidant capacity in healthy men and in men with coronary heart disease. Biomed Res Int 2014:216964. https://doi.org/10.1155/2014/216964
Giannakakis A, Zhang J, Jenjaroenpun P, Nama S, Zainolabidin N, Aau MY, Yarmishyn AA, Vaz C, Ivshina AV, Grinchuk OV, Voorhoeve M, Vardy LA, Sampath P, Kuznetsov VA, Kurochkin IV, Guccione E (2015) Contrasting expression patterns of coding and noncoding parts of the human genome upon oxidative stress. Sci Rep 5:9737. https://doi.org/10.1038/srep09737
Gimbrone MA Jr, García-Cardeña G (2016) Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res 118(4):620–636. https://doi.org/10.1161/CIRCRESAHA.115.306301
Goldstein LB, Bushnell CD, Adams RJ, Appel LJ, Braun LT, Chaturvedi S, Creager MA, Culebras A, Eckel RH, Hart RG, Hinchey JA, Howard VJ, Jauch EC, Levine SR, Meschia JF, Moore WS, Nixon JV, Pearson TA, American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Epidemiology and Prevention; Council for High Blood Pressure Research,; Council on Peripheral Vascular Disease, and Interdisciplinary Council on Quality of Care and Outcomes Research (2011) Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 42(2):517–584. https://doi.org/10.1161/STR.0b013e3181fcb238
Gong YY, Luo JY, Wang L, Huang Y (2018) MicroRNAs regulating reactive oxygen species in cardiovascular diseases. Antioxid Redox Signal 29(11):1092–1107. https://doi.org/10.1089/ars.2017.7328
Grande I, Magalhães PV, Kunz M, Vieta E, Kapczinski F (2012) Mediators of allostasis and systemic toxicity in bipolar disorder. Physiol Behav 106(1):46–50. https://doi.org/10.1016/j.physbeh.2011.10.029
Grant R (2010) Resveratrol increases intracellular NAD+ levels through up regulation of the NAD+ synthetic enzyme nicotinamide mononucleotide adenylyltransferase. Nat Precis. https://doi.org/10.1038/npre.2010.4421.1
Griesser E, Vemula V, Raulien N, Wagner U, Reeg S, Grune T, Fedorova M (2017) Cross-talk between lipid and protein carbonylation in a dynamic cardiomyocyte model of mild nitroxidative stress. Redox Biol 11:438–455. https://doi.org/10.1016/j.redox.2016.12.028
Guarente L (2013) Calorie restriction and sirtuins revisited. Genes Dev 27(19):2072–2085. https://doi.org/10.1101/gad.227439.113
Guo J, Lu L, Hua Y, Huang K, Wang I, Huang L, Fu Q, Chen A, Chan P, Fan H, Liu ZM, Wang BH (2017) Vasculopathy in the setting of cardiorenal syndrome: roles of protein-bound uremic toxins. Am J Physiol Heart Circ Physiol 313(1):H1–H13. https://doi.org/10.1152/ajpheart.00787
Guo J, Pereira TJ, Mori Y, Gonzalez Medina M, Breen DM, Dalvi PS, Zhang H, McCole DF, McBurney MW, Heximer SP, Tsiani EL, Dolinsky VW, Giacca A (2020) Resveratrol inhibits neointimal growth after arterial injury in high-fat-fed rodents: the roles of SIRT1 and AMPK. J Vasc Res 57(6):325–340. https://doi.org/10.1159/000509217
Gupta SC, Singh R, Asters M, Liu J, Zhang X, Pabbidi MR, Watabe K, Mo YY (2016) Regulation of breast tumorigenesis through acid sensors. Oncogene 35(31):4102–4111. https://doi.org/10.1038/onc.2015.477
Hai Z, Zuo W (2016) Aberrant DNA methylation in the pathogenesis of atherosclerosis. Clin Chim Acta 456:69–74. https://doi.org/10.1016/j.cca.2016.02.026
Halade GV, Kain V (2017) Obesity and cardiometabolic defects in heart failure pathology. Compr Physiol 7(4):1463–1477. https://doi.org/10.1002/cphy.c170011
Han JH, Oh TJ, Lee G, Maeng HJ, Lee DH, Kim KM, Choi SH, Jang HC, Lee HS, Park KS, Kim YB, Lim S (2017) The beneficial effects of empagliflozin, an SGLT2 inhibitor, on atherosclerosis in ApoE -/- mice fed a western diet. Diabetologia. 60(2):364–376. https://doi.org/10.1007/s00125-016-4158-2
Handy DE, Castro R, Loscalzo J (2011) Epigenetic modifications: basic mechanisms and role in cardiovascular disease. Circulation. 123(19):2145–2156. https://doi.org/10.1161/CIRCULATIONAHA.110.956839
Hanske J, Toffey JR, Morenz AM, Bonilla AJ, Schiavoni KH, Pletneva EV (2012) Conformational properties of cardiolipin-bound cytochrome c. Proc Natl Acad Sci U S A 109(1):125–130. https://doi.org/10.1073/pnas.1112312108
Harper JW, Ordureau A, Heo JM (2018) Building and decoding ubiquitin chains for mitophagy. Nat Rev Mol Cell Biol 19(2):93–108. https://doi.org/10.1038/nrm.2017.129
He F, Zuo L (2015) Redox roles of reactive oxygen species in cardiovascular diseases. Int J Mol Sci 16(11):27770–27780. https://doi.org/10.3390/ijms161126059
Heiss EH, Dirsch VM (2014) Regulation of eNOS enzyme activity by posttranslational modification. Curr Pharm Des 20(22):3503–3513. https://doi.org/10.2174/13816128113196660745
Heiss C, Keen CL, Kelm M (2010) Flavanols and cardiovascular disease prevention. Eur Heart J 31(21):2583–2592. https://doi.org/10.1093/eurheartj/ehq332
Ho E, Karimi Galougahi K, Liu CC, Bhindi R, Figtree GA (2013) Biological markers of oxidative stress: applications to cardiovascular research and practice. Redox Biol 1(1):483–491. https://doi.org/10.1016/j.redox.2013.07.006
Holloszy JO (2011) Regulation of mitochondrial biogenesis and GLUT4 expression by exercise. Compr Physiol 1(2):921–940. https://doi.org/10.1002/cphy.c100052
Holterman CE, Thibodeau JF, Kennedy CR (2015) NADPH oxidase 5 and renal disease. Curr Opin Nephrol Hypertens 24(1):81–87. https://doi.org/10.1097/MNH.0000000000000081
Howson JMM, Zhao W, Barnes DR, Ho WK, Young R, Paul DS, Waite LL, Freitag DF, Fauman EB, Salfati EL, Sun BB, Eicher JD, Johnson AD, WHH S, Nielsen SF, Lin WY, Surendran P, Malarstig A, Wilk JB, Tybjærg-Hansen A, Rasmussen KL, Kamstrup PR, Deloukas P, Erdmann J, Kathiresan S, Samani NJ, Schunkert H, Watkins H, CARDIoGRAMplusC4D, Do R, Rader DJ, Johnson JA, Hazen SL, Quyyumi AA, Spertus JA, Pepine CJ, Franceschini N, Justice A, Reiner AP, Buyske S, Hindorff LA, Carty CL, North KE, Kooperberg C, Boerwinkle E, Young K, Graff M, Peters U, Absher D, Hsiung CA, Lee WJ, Taylor KD, Chen YH, Lee IT, Guo X, Chung RH, Hung YJ, Rotter JI, Juang JJ, Quertermous T, Wang TD, Rasheed A, Frossard P, Alam DS, Majumder AAS, Di Angelantonio E, Chowdhury R, EPIC-CVD, Chen YI, Nordestgaard BG, Assimes TL, Danesh J, Butterworth AS, Saleheen D (2017) Fifteen new risk loci for coronary artery disease highlight arterial-wall-specific mechanisms. Nat Genet 49(7):1113–1119. https://doi.org/10.1038/ng.3874
Hsu CP, Zhai P, Yamamoto T, Maejima Y, Matsushima S, Hariharan N, Shao D, Takagi H, Oka S, Sadoshima J (2010) Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation. 122(21):2170–2182. https://doi.org/10.1161/CIRCULATIONAHA.110.958033
Huertas-Vazquez A, Leon-Mimila P, Wang J (2019) Relevance of multi-omics studies in cardiovascular diseases. Front Cardiovasc Med 6:91. https://doi.org/10.3389/fcvm.2019.00091
Huffman JC, Celano CM, Beach SR, Motiwala SR, Januzzi JL (2013) Depression and cardiac disease: epidemiology, mechanisms, and diagnosis. Cardiovasc Psychiatry Neurol 2013:695925. https://doi.org/10.1155/2013/695925
Hulsmans M, Holvoet P (2010) The vicious circle between oxidative stress and inflammation in atherosclerosis. J Cell Mol Med 14(1-2):70–78. https://doi.org/10.1111/j.1582-4934.2009.00978.x
Hwang JW, Yao H, Caito S, Sundar IK, Rahman I (2013) Redox regulation of SIRT1 in inflammation and cellular senescence. Free Radic Biol Med 61:95–110. https://doi.org/10.1016/j.freeradbiomed.2013.03.015
Il’yasova D, Fontana L, Bhapkar M, Pieper CF, Spasojevic I, Redman LM, Das SK, Huffman KM, Kraus WE, CALERIE Study Investigators (2018) Effects of 2 years of caloric restriction on oxidative status assessed by urinary F2-isoprostanes: The CALERIE 2 randomized clinical trial. Aging Cell 17(2):e12719. https://doi.org/10.1111/acel.12719
Incalza MA, D'Oria R, Natalicchio A, Perrini S, Laviola L, Giorgino F (2018) Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc Pharmacol 100:1–19. https://doi.org/10.1016/j.vph.2017.05.005
Ishigami J, Grams ME, Naik RP, Coresh J, Matsushita K (2016) Chronic kidney disease and risk for gastrointestinal bleeding in the community: the Atherosclerosis Risk in Communities (ARIC) Study. Clin J Am Soc Nephrol 11(10):1735–1743. https://doi.org/10.2215/CJN.02170216
JCS Joint Working Group (2014) Guidelines for diagnosis and treatment of patients with vasospastic angina (Coronary Spastic Angina) (JCS 2013). Circ J 78(11):2779–2801. https://doi.org/10.1253/circj.cj-66-0098
Jiang S, Yang Y, Li T, Ma Z, Hu W, Deng C, Fan C, Lv J, Sun Y, Yi W (2016) An overview of the mechanisms and novel roles of Nrf2 in cardiovascular diseases. Expert Opin Ther Targets 20(12):1413–1424. https://doi.org/10.1080/14728222.2016.1250887
Joergensen A, Broedbaek K, Weimann A, Semba RD, Ferrucci L, Joergensen MB, Poulsen HE (2011) Association between urinary excretion of cortisol and markers of oxidatively damaged DNA and RNA in humans. PLoS One 6(6):e20795. https://doi.org/10.1371/journal.pone.0020795
Johnson RJ, Nakagawa T, Jalal D, Sánchez-Lozada LG, Kang DH, Ritz E (2013) Uric acid and chronic kidney disease: which is chasing which? Nephrol Dial Transplant 28(9):2221–2228. https://doi.org/10.1093/ndt/gft029
Johnson SA, Feresin RG, Navaei N, Figueroa A, Elam ML, Akhavan NS, Hooshmand S, Pourafshar S, Payton ME, Arjmandi BH (2017) Effects of daily blueberry consumption on circulating biomarkers of oxidative stress, inflammation, and antioxidant defense in postmenopausal women with pre- and stage 1-hypertension: a randomized controlled trial. Food Funct 8(1):372–380. https://doi.org/10.1039/c6fo01216g
Johnson RJ, Bakris GL, Borghi C, Chonchol MB, Feldman D, Lanaspa MA, Merriman TR, Moe OW, Mount DB, Sanchez Lozada LG, Stahl E, Weiner DE, Chertow GM (2018) Hyperuricemia, acute and chronic kidney disease, hypertension, and cardiovascular disease: report of a Scientific Workshop Organized by the National Kidney Foundation. Am J Kidney Dis 71(6):851–865. https://doi.org/10.1053/j.ajkd.2017.12.009
Junior RF, Dabkowski ER, Shekar KC, O Connell KA, Hecker PA, Murphy MP (2018) MitoQ improves mitochondrial dysfunction in heart failure induced by pressure overload. Free Radic Biol Med 117:18–29. https://doi.org/10.1016/j.freeradbiomed.2018.01.012
Katerji M, Filippova M, Duerksen-Hughes P (2019) Approaches and methods to measure oxidative stress in clinical samples: research applications in the cancer field. Oxidative Med Cell Longev 2019:1279250. https://doi.org/10.1155/2019/1279250
Katkam SK, Indumathi B, Naushad SM, Kutala VK (2019a) Impact of genetic and epigenetic factors on the oxidative stress in cardiovascular disease. In: Modulation of Oxidative Stress in Heart Disease. Springer, pp 107-128
Katkam SK, Indumathi B, Naushad SM, Kutala VK (2019b) Impact of genetic and epigenetic factors on the oxidative stress in cardiovascular disease modulation of oxidative stress in heart disease, p 107-128
Kaur N, Singh J, Reddy S (2018) Interaction between ALOX15 polymorphisms and coronary artery disease in North Indian population. Clin Exp Hypertens 40(4):398–405. https://doi.org/10.1080/10641963.2017.1384485
Kawabata K, Yoshioka Y, Terao J (2019) Role of intestinal microbiota in the bioavailability and physiological functions of dietary polyphenols. Molecules 24(2):370. https://doi.org/10.3390/molecules24020370
Kay CD, Hooper L, Kroon PA, Rimm EB, Cassidy A (2012) Relative impact of flavonoid composition, dose and structure on vascular function: a systematic review of randomised controlled trials of flavonoid-rich food products. Mol Nutr Food Res 56(11):1605–1616. https://doi.org/10.1002/mnfr.201200363
Kaypee S, Sudarshan D, Shanmugam MK, Mukherjee D, Sethi G, Kundu TK (2016) Aberrant lysine acetylation in tumorigenesis: implications in the development of therapeutics. Pharmacol Ther 162:98–119. https://doi.org/10.1016/j.pharmthera.2016.01.011
Kemp JR, Unal H, Desnoyer R, Yue H, Bhatnagar A, Karnik SS (2014) Angiotensin II-regulated microRNA 483-3p directly targets multiple components of the renin-angiotensin system. J Mol Cell Cardiol 75:25–39. https://doi.org/10.1016/j.yjmcc.2014.06.008
Khamis AA, Salama AF, Kenawy ME, Mohamed TM (2017) Regulation of hepatic hydroxy methyl glutarate - CoA reductase for controlling hypercholesterolemia in rats. Biomed Pharmacother 95:1242–1250. https://doi.org/10.1016/j.biopha.2017.09.071
Kida Y, Goligorsky MS (2016) Sirtuins, cell senescence, and vascular aging. Can J Cardiol 32(5):634–641. https://doi.org/10.1016/j.cjca.2015.11.022
Kim CS, Kim YR, Naqvi A, Kumar S, Hoffman TA, Jung SB, Kumar A, Jeon BH, McNamara DM, Irani K (2011) Homocysteine promotes human endothelial cell dysfunction via site-specific epigenetic regulation of p66shc. Cardiovasc Res 92(3):466–475. https://doi.org/10.1093/cvr/cvr250
Kim GH, Ryan JJ, Archer SL (2013) The role of redox signaling in epigenetics and cardiovascular disease. Antioxid Redox Signal 18(15):1920–1936. https://doi.org/10.1089/ars.2012.4926
Kivimäki M, Steptoe A (2018) Effects of stress on the development and progression of cardiovascular disease. Nat Rev Cardiol 15(4):215–229. https://doi.org/10.1038/nrcardio.2017.189
Klarin D, Zhu QM, Emdin CA, Chaffin M, Horner S, BJ MM, Leed A, Weale ME, Spencer CCA, Aguet F, Segrè AV, Ardlie KG, Khera AV, Kaushik VK, Natarajan P, CARDIoGRAMplusC4D Consortium, Kathiresan S (2017) Genetic analysis in UK Biobank links insulin resistance and transendothelial migration pathways to coronary artery disease. Nat Genet 49(9):1392–1397. https://doi.org/10.1038/ng.3914
Klaunig JE, Wang Z, Pu X, Zhou S (2011) Oxidative stress and oxidative damage in chemical carcinogenesis. Toxicol Appl Pharmacol 254(2):86–99. https://doi.org/10.1016/j.taap.2009.11.028
Kleniewska P, Piechota A, Skibska B, Gorąca A (2012) The NADPH oxidase family and its inhibitors. Arch Immunol Ther Exp (Warsz) 60(4):277–294. https://doi.org/10.1007/s00005-012-0176-z
Koçak S, Harmankaya A, Erdemir E, Dündar ZD, Kesli R, Alibasic H (2016) Association of severity of coronary lesion with markers of acute infection and inflammation in patients with acute coronary syndrome. Eur J Emerg Med 15:157. https://doi.org/10.5152/eajem.2016.79188
Koene RJ, Prizment AE, Blaes A, Konety SH (2016) Shared risk factors in cardiovascular disease and cancer. Circulation. 133(11):1104–1114. https://doi.org/10.1161/CIRCULATIONAHA.115.020406
Kokkinos P, Myers J (2010) Exercise and physical activity: clinical outcomes and applications. Circulation 122(16):1637–1648. https://doi.org/10.1161/CIRCULATIONAHA.110.948349
Kowalska K, Socha E, Milnerowicz H (2015) Review: the role of paraoxonase in cardiovascular diseases. Ann Clin Lab Sci 45(2):226–233
Koyama K (2014) Exercise-induced oxidative stress: a tool for “hormesis” and “adaptive response”. J Phys Fitness Sports Med 3:115–120. https://doi.org/10.7600/jpfsm.3.115
Kreutzer FP, Fiedler J, Thum T (2020) Non-coding RNAs: key players in cardiac disease. J Physiol 598(14):2995–3003. https://doi.org/10.1113/JP278131
Kruger MJ, Davies N, Myburgh KH, Lecour S (2014) Proanthocyanidins, anthocyanins and cardiovascular diseases. Int Food Res J 59:41–52. https://doi.org/10.1016/j.foodres.2014.01.046
Kukongviriyapan U, Sompamit K, Pannangpetch P, Kukongviriyapan V, Donpunha W (2012) Preventive and therapeutic effects of quercetin on lipopolysaccharide-induced oxidative stress and vascular dysfunction in mice. Can J Physiol Pharmacol 90(10):1345–1353. https://doi.org/10.1139/y2012-101
Kung JT, Colognori D, Lee JT (2013) Long noncoding RNAs: past, present, and future. Genetics. 193(3):651–669. https://doi.org/10.1534/genetics.112.146704
Kunutsor SK, Spee JM, Kieneker LM, Gansevoort RT, Dullaart RPF, Voerman AJ, Touw DJ, Bakker SJL (2018) Self-reported smoking, urine cotinine, and risk of cardiovascular disease: findings from the PREVEND (Prevention of Renal and Vascular End-Stage Disease) Prospective Cohort Study. J Am Heart Assoc 7(10):e008726. https://doi.org/10.1161/JAHA.118.008726
Kuo CF, See LC, Yu KH, Chou IJ, Chiou MJ, Luo SF (2013) Significance of serum uric acid levels on the risk of all-cause and cardiovascular mortality. Rheumatology (Oxford) 52(1):127–134. https://doi.org/10.1093/rheumatology/kes223
Kura B, Szeiffova Bacova B, Kalocayova B, Sykora M, Slezak J (2020) Oxidative stress-responsive microRNAs in heart injury. Int J Mol Sci 21(1):358. https://doi.org/10.3390/ijms21010358
Kwekkeboom RF, Lei Z, Doevendans PA, Musters RJ, Sluijter JP (2014) Targeted delivery of miRNA therapeutics for cardiovascular diseases: opportunities and challenges. Clin Sci (Lond) 127(6):351–365. https://doi.org/10.1042/CS20140005
Lassègue B, San Martín A, Griendling KK (2012) Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ Res 110(10):1364–1390. https://doi.org/10.1161/CIRCRESAHA.111.243972
Lee R, Margaritis M, Channon KM, Antoniades C (2012) Evaluating oxidative stress in human cardiovascular disease: methodological aspects and considerations. Curr Med Chem 19(16):2504–2520. https://doi.org/10.2174/092986712800493057
Lee JH, Guo Z, Myler LR, Zheng S, Paull TT (2014) Direct activation of ATM by resveratrol under oxidizing conditions. PLoS One 9(6):e97969. https://doi.org/10.1371/journal.pone.0097969
Lee PY, Chin SF, Low TY, Jamal R (2018) Probing the colorectal cancer proteome for biomarkers: current status and perspectives. J Proteome 187:93–105. https://doi.org/10.1016/j.jprot.2018.06.014
Leopold JA (2015) Antioxidants and coronary artery disease: from pathophysiology to preventive therapy. Coron Artery Dis 26(2):176–183. https://doi.org/10.1097/MCA.0000000000000187
Leufkens AM, van Duijnhoven FJ, Woudt SH, Siersema PD, Jenab M, Jansen EH, Pischon T, Tjønneland A, Olsen A, Overvad K, Boutron-Ruault MC, Clavel-Chapelon F, Morois S, Palli D, Pala V, Tumino R, Vineis P, Panico S, Kaaks R, Lukanova A, Boeing H, Aleksandrova K, Trichopoulou A, Trichopoulos D, Dilis V, Peeters PH, Skeie G, González CA, Argüelles M, Sánchez MJ, Dorronsoro M, Huerta JM, Ardanaz E, Hallmans G, Palmqvist R, Khaw KT, Wareham N, Allen NE, Crowe FL, Fedirko V, Norat T, Riboli E, Bueno-de-Mesquita HB (2012) Biomarkers of oxidative stress and risk of developing colorectal cancer: a cohort-nested case-control study in the European Prospective Investigation Into Cancer and Nutrition. Am J Epidemiol 175(7):653–663. https://doi.org/10.1093/aje/kwr418
Li L, Zhang HN, Chen HZ, Gao P, Zhu LH, Li HL, Lv X, Zhang QJ, Zhang R, Wang Z, She ZG, Zhang R, Wei YS, Du GH, Liu DP, Liang CC (2011) SIRT1 acts as a modulator of neointima formation following vascular injury in mice. Circ Res 108(10):1180–1189. https://doi.org/10.1161/CIRCRESAHA.110.237875
Li Y, Chen X, Li P, Xiao Q, Hou D, Kong X (2020) CD47 antibody suppresses isoproterenol-induced cardiac hypertrophy through activation of autophagy. Am J Transl Res 12(9):5908–5923
Lichtman JH, Froelicher ES, Blumenthal JA, Carney RM, Doering LV, Frasure-Smith N, Freedland KE, Jaffe AS, Leifheit-Limson EC, Sheps DS, Vaccarino V, Wulsin L (2014) American Heart Association Statistics Committee of the Council on Epidemiology and Prevention and the Council on Cardiovascular and Stroke Nursing. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations: a scientific statement from the American Heart Association. Circulation 25;129(12):1350-69. https://doi.org/10.1161/CIR.0000000000000019
Libby P, Okamoto Y, Rocha VZ, Folco E (2010) Inflammation in atherosclerosis: transition from theory to practice. Circ J 74(2):213–220. https://doi.org/10.1253/circj.cj-09-0706
Libby P, Ridker PM, Hansson GK (2011) Progress and challenges in translating the biology of atherosclerosis. Nature 473(7347):317–325. https://doi.org/10.1038/nature10146
Lim CSH, Lim SL (2013) Ferric reducing capacity versus ferric reducing antioxidant power for measuring total antioxidant capacity. Lab Med 44:51–55. https://doi.org/10.1309/LM93W7KTFNPZIXRR
Liu M, Li X, Lu L, Cao Y, Sun R, Chen S, Zhang P (2014) Cardiovascular disease and its relationship with chronic kidney disease. Eur Rev Med Pharmacol Sci 18:2918–2926
Liu D, Liu L, Song Z, Hu Z, Liu J, Hou D (2017a) Genetic variations of oxidative stress related genes ALOX5, ALOX5AP and MPO modulate ischemic stroke susceptibility through main effects and epistatic interactions in a Chinese population. Cell Physiol Biochem 43(4):1588–1602. https://doi.org/10.1159/000482023
Liu H, Xu Z, Sun C, Gu D, Teng X, Zhao Y, Zheng Z (2017b) A variant in COX-2 gene is associated with left main coronary artery disease and clinical outcomes of coronary artery bypass grafting. Biomed Res Int 2017:2924731. https://doi.org/10.1155/2017/2924731
Lobo V, Patil A, Phatak A, Chandra N (2010) Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev 4(8):118–126. https://doi.org/10.4103/0973-7847.70902
Loperena R, Harrison DG (2017) Oxidative stress and hypertensive diseases. Med Clin N Am 101(1):169–193. https://doi.org/10.1016/j.mcna.2016.08.004
Lowe F (2014) Biomarkers of oxidative stress. Syst Biol Free Radic Antioxid 2:65–87. https://doi.org/10.1007/978-3-642-30018-9_4
Lu H, Cassis LA, Kooi CW, Daugherty A (2016) Structure and functions of angiotensinogen. Hypertens Res 39(7):492–500. https://doi.org/10.1038/hr.2016.17
Lüersen K, Schmelzer C, Boesch-Saadatmandi C, Kohl C, Rimbach G, Döring F (2011) Paraoxonase 1 polymorphism Q192R affects the pro-inflammatory cytokine TNF-alpha in healthy males. BMC Res Notes 4:141. https://doi.org/10.1186/1756-0500-4-141
Lyngdoh T, Marques-Vidal P, Paccaud F, Preisig M, Waeber G, Bochud M, Vollenweider P (2011) Elevated serum uric acid is associated with high circulating inflammatory cytokines in the population-based Colaus study. PLoS One 6(5):e19901. https://doi.org/10.1371/journal.pone.0019901
Ma Q (2010) Transcriptional responses to oxidative stress: pathological and toxicological implications. Pharmacol Ther 125(3):376–393. https://doi.org/10.1016/j.pharmthera.2009.11.004
Ma Y, Peng D, Liu C, Huang C, Luo J (2017) Serum high concentrations of homocysteine and low levels of folic acid and vitamin B12 are significantly correlated with the categories of coronary artery diseases. BMC Cardiovasc Disord 17(1):37. https://doi.org/10.1186/s12872-017-0475-8
Mangge H, Weghuber D, Prassl R, Haara A, Schnedl W, Postolache TT, Fuchs D (2015) The role of vitamin D in atherosclerosis inflammation revisited: more a bystander than a player? Curr Vasc Pharmacol 13(3):392–398. https://doi.org/10.2174/1570161111666131209125454
Maron BA, Tang SS, Loscalzo J (2013) S-nitrosothiols and the S-nitrosoproteome of the cardiovascular system. Antioxid Redox Signal 18(3):270–287. https://doi.org/10.1089/ars.2012.4744
Martínez GJ, Celermajer DS, Patel S (2018) The NLRP3 inflammasome and the emerging role of colchicine to inhibit atherosclerosis-associated inflammation. Atherosclerosis. 269:262–271. https://doi.org/10.1016/j.atherosclerosis.2017.12.027
Martín-Timón I, Sevillano-Collantes C, Segura-Galindo A, Del Cañizo-Gómez FJ (2014) Type 2 diabetes and cardiovascular disease: have all risk factors the same strength? World J Diabetes 5(4):444–470. https://doi.org/10.4239/wjd.v5.i4.444
Mazaheri M, Karimian M, Behjati M, Raygan F, Hosseinzadeh CA (2017 Nov) Association analysis of rs1049255 and rs4673 transitions in p22phox gene with coronary artery disease: a case-control study and a computational analysis. Ir J Med Sci 186(4):921–928. https://doi.org/10.1007/s11845-017-1601-4
Mazidi M, Toth PP, Banach M (2018) C-reactive protein is associated with prevalence of the metabolic syndrome, hypertension, and diabetes mellitus in US adults. Angiology. 69(5):438–442. https://doi.org/10.1177/0003319717729288
McCullough ML, Peterson JJ, Patel R, Jacques PF, Shah R, Dwyer JT (2012) Flavonoid intake and cardiovascular disease mortality in a prospective cohort of US adults. Am J Clin Nutr 95(2):454–464. https://doi.org/10.3945/ajcn.111.016634
Meghatria F, Belhamiti O (2021) Predictive model for the risk of cardiovascular disease and type 2 diabetes in obese people. Chaos, Solitons Fractals 146:110834. https://doi.org/10.1016/j.chaos.2021.110834
Mehta MM, Weinberg SE, Chandel NS (2017) Mitochondrial control of immunity: beyond ATP. Nat Rev Immunol 17(10):608–620. https://doi.org/10.1038/nri.2017.66
Members WC et al (2010) 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Circulation 122(25):e584–e636. https://doi.org/10.1161/CIR.0b013e3182051b4c
Meneses MJ, Silvestre R, Sousa-Lima I, Macedo MP (2019) Paraoxonase-1 as a regulator of glucose and lipid homeostasis: impact on the onset and progression of metabolic disorders. Int J Mol Sci 20(16):4049. https://doi.org/10.3390/ijms20164049
Merry TL, Ristow M (2016) Do antioxidant supplements interfere with skeletal muscle adaptation to exercise training? J Physiol 594(18):5135–5147. https://doi.org/10.1113/JP270654
Messner B, Bernhard D (2014) Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb Vasc Biol 34(3):509–515. https://doi.org/10.1161/ATVBAHA.113.300156
Meydani SN, Das SK, Pieper CF, Lewis MR, Klein S, Dixit VD, Gupta AK, Villareal DT, Bhapkar M, Huang M, Fuss PJ, Roberts SB, Holloszy JO, Fontana L (2016) Long-term moderate calorie restriction inhibits inflammation without impairing cell-mediated immunity: a randomized controlled trial in non-obese humans. Aging (Albany NY) 8(7):1416–1431. https://doi.org/10.18632/aging.100994
Milagro FI, Mansego ML, De Miguel C, Martínez JA (2013) Dietary factors, epigenetic modifications and obesity outcomes: progresses and perspectives. Mol Asp Med 34(4):782–812. https://doi.org/10.1016/j.mam.2012.06.010
Mishra AK, Driessen NN, Appelmelk BJ, Besra GS (2011) Lipoarabinomannan and related glycoconjugates: structure, biogenesis and role in Mycobacterium tuberculosis physiology and host-pathogen interaction. FEMS Microbiol Rev 35(6):1126–1157. https://doi.org/10.1111/j.1574-6976.2011.00276.x
Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB (2014) Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal 20(7):1126–1167. https://doi.org/10.1089/ars.2012.5149
Montezano AC, Touyz RM (2014) Reactive oxygen species, vascular Noxs, and hypertension: focus on translational and clinical research. Antioxid Redox Signal 20(1):164–182. https://doi.org/10.1089/ars.2013.5302
Montezano AC, Dulak-Lis M, Tsiropoulou S, Harvey A, Briones AM, Touyz RM (2015) Oxidative stress and human hypertension: vascular mechanisms, biomarkers, and novel therapies. Can J Cardiol 31(5):631–641. https://doi.org/10.1016/j.cjca.2015.02.008
Morrison D, Hughes J, Della Gatta PA, Mason S, Lamon S, Russell AP, Wadley GD (2015) Vitamin C and E supplementation prevents some of the cellular adaptations to endurance-training in humans. Free Radic Biol Med 89:852–862. https://doi.org/10.1016/j.freeradbiomed.2015.10.412
Most J, Tosti V, Redman LM, Fontana L (2017) Calorie restriction in humans: an update. Ageing Res Rev 39:36–45. https://doi.org/10.1016/j.arr.2016.08.005
Mozaffarian D, Wu JH (2011) Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol 58(20):2047–2067. https://doi.org/10.1016/j.jacc.2011.06.063
Mudau M, Genis A, Lochner A, Strijdom H (2012) Endothelial dysfunction: the early predictor of atherosclerosis. Cardiovasc J Afr 23(4):222–231. https://doi.org/10.5830/CVJA-2011-068
Murphy RB, Tommasi S, Lewis BC, Mangoni AA (2016) Inhibitors of the hydrolytic enzyme dimethylarginine dimethylaminohydrolase (DDAH): discovery, synthesis and development. Molecules 21(5):615. https://doi.org/10.3390/molecules21050615
Nakamura K, Fuster JJ, Walsh K (2014) Adipokines: a link between obesity and cardiovascular disease. J Cardiol 63(4):250–259. https://doi.org/10.1016/j.jjcc.2013.11.006
Nardinocchi L, Pantisano V, Puca R, Porru M, Aiello A, Grasselli A, Leonetti C, Safran M, Rechavi G, Givol D, Farsetti A, D'Orazi G (2010) Zinc downregulates HIF-1α and inhibits its activity in tumor cells in vitro and in vivo. PLoS One 5(12):e15048. https://doi.org/10.1371/journal.pone.0015048
Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR, CANVAS Program Collaborative Group (2017) Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 377(7):644–657. https://doi.org/10.1056/NEJMoa1611925
Neri M, Fineschi V, Di Paolo M, Pomara C, Riezzo I, Turillazzi E, Cerretani D (2015) Cardiac oxidative stress and inflammatory cytokines response after myocardial infarction. Curr Vasc Pharmacol 13(1):26–36. https://doi.org/10.2174/15701611113119990003
Neves AL, Mohammedi K, Emery N, Roussel R, Fumeron F, Marre M, Velho G (2012) Allelic variations in superoxide dismutase-1 (SOD1) gene and renal and cardiovascular morbidity and mortality in type 2 diabetic subjects. Mol Genet Metab 106(3):359–365. https://doi.org/10.1016/j.ymgme.2012.04.023
Nimse SB, Pal D (2015) Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv 5:27986–28006. https://doi.org/10.1039/C4RA13315C
Nocella C, Cammisotto V, Pigozzi F, Borrione P, Fossati C, D'Amico A, Cangemi R, Peruzzi M, Gobbi G, Ettorre E, Frati G, Cavarretta E, Carnevale R, SMiLe Group (2019) Impairment between oxidant and antioxidant systems: short- and long-term implications for athletes' health. Nutrients. 11(6):1353. https://doi.org/10.3390/nu11061353
Nordestgaard BG, Varbo A (2014) Triglycerides and cardiovascular disease. Lancet 384(9943):626–635. https://doi.org/10.1016/S0140-6736(14)61177-6
Nozik-Grayck E, Woods C, Stearman RS, Venkataraman S, Ferguson BS, Swain K, Bowler RP, Geraci MW, Ihida-Stansbury K, Stenmark KR, McKinsey TA, Domann FE (2016) Histone deacetylation contributes to low extracellular superoxide dismutase expression in human idiopathic pulmonary arterial hypertension. Am J Phys Lung Cell Mol Phys 311(1):L124–L134. https://doi.org/10.1152/ajplung.00263.2015
Nuhu F, Bhandari S (2018) Oxidative stress and cardiovascular complications in chronic kidney disease, the impact of anaemia. Pharmaceuticals (Basel) 11(4):103. https://doi.org/10.3390/ph11040103
Nuhu F, Seymour AM, Bhandari S (2019) Impact of intravenous iron on oxidative stress and mitochondrial function in experimental chronic kidney disease. Antioxidants (Basel) 8(10):498. https://doi.org/10.3390/antiox8100498
Oelze M, Kröller-Schön S, Welschof P, Jansen T, Hausding M, Mikhed Y, Stamm P, Mader M, Zinßius E, Agdauletova S, Gottschlich A, Steven S, Schulz E, Bottari SP, Mayoux E, Münzel T, Daiber A (2014) The sodium-glucose co-transporter 2 inhibitor empagliflozin improves diabetes-induced vascular dysfunction in the streptozotocin diabetes rat model by interfering with oxidative stress and glucotoxicity. PLoS One 9(11):e112394. https://doi.org/10.1371/journal.pone.0112394
Oliveira-Paula GH, Lacchini R, Tanus-Santos JE (2017) Clinical and pharmacogenetic impact of endothelial nitric oxide synthase polymorphisms on cardiovascular diseases. Nitric Oxide 63:39–51. https://doi.org/10.1016/j.niox.2016.08.004
Oni-Orisan A, Deng Y, Schuck RN, Theken KN, Edin ML, Lih FB, Molnar K, DeGraff L, Tomer KB, Zeldin DC, Lee CR (2013) Dual modulation of cyclooxygenase and CYP epoxygenase metabolism and acute vascular inflammation in mice. Prostaglandins Other Lipid Mediat 104-105:67–73. https://doi.org/10.1016/j.prostaglandins.2012.09.003
Ouchi N, Parker JL, Lugus JJ, Walsh K (2011) Adipokines in inflammation and metabolic disease. Nat Rev Immunol 11(2):85–97. https://doi.org/10.1038/nri2921
Parthasarathy S, Raghavamenon A, Garelnabi MO, Santanam N (2010) Oxidized low-density lipoprotein. Methods Mol Biol 610:403–417. https://doi.org/10.1007/978-1-60327-029-8_24
Pelliccia F, Kaski JC, Crea F, Camici PG (2017) Pathophysiology of Takotsubo Syndrome. Circulation 135(24):2426–2441. https://doi.org/10.1161/CIRCULATIONAHA.116.027121
Peng JR, Lu TT, Chang HT, Ge X, Huang B, Li WM (2016) Elevated levels of plasma superoxide dismutases 1 and 2 in patients with coronary artery disease. Biomed Res Int 2016:3708905. https://doi.org/10.1155/2016/3708905
Peoples JN, Saraf A, Ghazal N, Pham TT, Kwong JQ (2019) Mitochondrial dysfunction and oxidative stress in heart disease. Exp Mol Med 51(12):1–13. https://doi.org/10.1038/s12276-019-0355-7
Peterson JJ, Dwyer JT, Jacques PF, McCullough ML (2012) Associations between flavonoids and cardiovascular disease incidence or mortality in European and US populations. Nutr Rev 70(9):491–508. https://doi.org/10.1111/j.1753-4887.2012.00508.x
Pisani A, Riccio E, Sabbatini M, Andreucci M, Del Rio A, Visciano B (2015) Effect of oral liposomal iron versus intravenous iron for treatment of iron deficiency anaemia in CKD patients: a randomized trial. Nephrol Dial Transplant 30(4):645–652. https://doi.org/10.1093/ndt/gfu357
Pompeani N, Rybalka E, Latchman H, Murphy RM, Croft K, Hayes A (2014) Skeletal muscle atrophy in sedentary Zucker obese rats is not caused by calpain-mediated muscle damage or lipid peroxidation induced by oxidative stress. J Negat Results Biomed 13:19. https://doi.org/10.1186/s12952-014-0019-z
Ponnuswamy P, Schröttle A, Ostermeier E, Grüner S, Huang PL, Ertl G, Hoffmann U, Nieswandt B, Kuhlencordt PJ (2012) eNOS protects from atherosclerosis despite relevant superoxide production by the enzyme in apoE mice. PLoS One 7(1):e30193. https://doi.org/10.1371/journal.pone.0030193
Pourvali K, Abbasi M, Mottaghi A (2016) Role of superoxide dismutase 2 gene Ala16Val polymorphism and total antioxidant capacity in diabetes and its complications. Avicenna J Med Biotechnol 8(2):48–56
Powers SK, Smuder AJ, Kavazis AN, Hudson MB (2010) Experimental guidelines for studies designed to investigate the impact of antioxidant supplementation on exercise performance. Int J Sport Nutr Exerc Metab 20(1):2–14. https://doi.org/10.1123/ijsnem.20.1.2
Prats M, Font R, García C, Muñoz-Cortés M, Cabré C, Jariod M, Romeu M, Giralt M, Martinez-Vea A (2014) Oxidative stress markers in predicting response to treatment with ferric carboxymaltose in nondialysis chronic kidney disease patients. Clin Nephrol 81(6):419–426. https://doi.org/10.5414/CN108166
Prymont-Przyminska A, Zwolinska A, Sarniak A, Wlodarczyk A, Krol M, Nowak M, de Graft-Johnson J, Padula G, Bialasiewicz P, Markowski J, Rutkowski KP, Nowak D (2014) Consumption of strawberries on a daily basis increases the non-urate 2,2-diphenyl-1-picryl-hydrazyl (DPPH) radical scavenging activity of fasting plasma in healthy subjects. J Clin Biochem Nutr 55(1):48–55. https://doi.org/10.3164/jcbn.13-93
Pu LN, Zhao Z, Zhang YT (2012) Investigation on cardiovascular risk prediction using genetic information. IEEE Trans Inf Technol Biomed 16(5):795–808. https://doi.org/10.1109/TITB.2012.2205009
Puddu P, Puddu GM, Cravero E, Vizioli L, Muscari A (2012) Relationships among hyperuricemia, endothelial dysfunction and cardiovascular disease: molecular mechanisms and clinical implications. J Cardiol 59(3):235–242. https://doi.org/10.1016/j.jjcc.2012.01.013
Purushothaman S, Renuka Nair R, Harikrishnan VS, Fernandez AC (2011) Temporal relation of cardiac hypertrophy, oxidative stress, and fatty acid metabolism in spontaneously hypertensive rat. Mol Cell Biochem 351(1-2):59–64. https://doi.org/10.1007/s11010-011-0711-y
Radak Z, Torma F, Berkes I, Goto S, Mimura T, Posa A, Balogh L, Boldogh I, Suzuki K, Higuchi M, Koltai E (2019) Exercise effects on physiological function during aging. Free Radic Biol Med 132:33–41. https://doi.org/10.1016/j.freeradbiomed.2018.10.444
Rai H, Parveen F, Kumar S, Kapoor A, Sinha N (2014) Association of endothelial nitric oxide synthase gene polymorphisms with coronary artery disease: an updated meta-analysis and systematic review. PLoS One 9(11):e113363. https://doi.org/10.1371/journal.pone.0113363
Ramprasath T, Murugan PS, Kalaiarasan E, Gomathi P, Rathinavel A, Selvam GS (2012) Genetic association of Glutathione peroxidase-1 (GPx-1) and NAD(P)H:Quinone Oxidoreductase 1(NQO1) variants and their association of CAD in patients with type-2 diabetes. Mol Cell Biochem 361(1-2):143–150. https://doi.org/10.1007/s11010-011-1098-5
Rao K, Sethi K, Ischia J, Gibson L, Galea L, Xiao L, Yim M, Chang M, Papa N, Bolton D, Shulkes A, Baldwin GS, Patel O (2017) Protective effect of zinc preconditioning against renal ischemia reperfusion injury is dose dependent. PLoS One 12(7):e0180028. https://doi.org/10.1371/journal.pone.0180028
Rastogi A, Bhansali A (2017) SGLT2 inhibitors through the windows of EMPA-REG and CANVAS trials: a review. Diabetes Ther 8(6):1245–1251. https://doi.org/10.1007/s13300-017-0320-1
Ravipati AS, Zhang L, Koyyalamudi SR, Jeong SC, Reddy N, Bartlett J, Smith PT, Shanmugam K, Münch G, Wu MJ, Satyanarayanan M, Vysetti B (2012) Antioxidant and anti-inflammatory activities of selected Chinese medicinal plants and their relation with antioxidant content. BMC Complement Altern Med 12:173. https://doi.org/10.1186/1472-6882-12-173
Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB (2010) Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med 49(11):1603–1616. https://doi.org/10.1016/j.freeradbiomed.2010.09.006
Ricciotti E, FitzGerald GA (2011) Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol 31(5):986–1000. https://doi.org/10.1161/ATVBAHA.110.207449
Richter EA, Hargreaves M (2013) Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Rev 93(3):993–1017. https://doi.org/10.1152/physrev.00038.2012
Rifkin SB, Shrubsole MJ, Cai Q, Smalley WE, Ness RM, Swift LL, Milne G, Zheng W, Murff HJ (2021) Differences in erythrocyte phospholipid membrane long-chain polyunsaturated fatty acids and the prevalence of fatty acid desaturase genotype among African Americans and European Americans. Prostaglandins Leukot Essent Fat Acids 164:102216. https://doi.org/10.1016/j.plefa.2020.102216
Rizzi F, Conti C, Dogliotti E, Terranegra A, Salvi E, Braga D, Ricca F, Lupoli S, Mingione A, Pivari F, Brasacchio C, Barcella M, Chittani M, D'Avila F, Turiel M, Lazzaroni M, Soldati L, Cusi D, Barlassina C (2016) Interaction between polyphenols intake and PON1 gene variants on markers of cardiovascular disease: a nutrigenetic observational study. J Transl Med 14(1):186. https://doi.org/10.1186/s12967-016-0941-6
Rochette L, Zeller M, Cottin Y, Vergely C (2014) Diabetes, oxidative stress and therapeutic strategies. Biochim Biophys Acta 1840(9):2709–2729. https://doi.org/10.1016/j.bbagen.2014.05.017
Ros E (2010) Health benefits of nut consumption. Nutrients. 2(7):652–682. https://doi.org/10.3390/nu2070652
Rostoff P, Szczeklik W, Piwowarska W, Konduracka E, Sanak M, Nessler J (2014) Association of common cyclooxygenase-2 (COX-2) gene polymorphisms with clinical and angiographic characteristics of patients with coronary artery disease. Przegl Lek 71:314–318
Roy A, Rawal I, Jabbour S, Prabhakaran D (2017) Tobacco and cardiovascular disease: a summary of evidence. In: Cardiovascular, Respiratory, and Related Disorders. 3rd edition. The International Bank for Reconstruction and Development/The World Bank
Rubio CP, Hernández-Ruiz J, Martinez-Subiela S, Tvarijonaviciute A, Ceron JJ (2016) Spectrophotometric assays for total antioxidant capacity (TAC) in dog serum: an update. BMC Vet Res 12(1):166. https://doi.org/10.1186/s12917-016-0792-7
Rückert IM, Schunk M, Holle R, Schipf S, Völzke H, Kluttig A, Greiser KH, Berger K, Müller G, Ellert U, Neuhauser H, Rathmann W, Tamayo T, Moebus S, Andrich S, Meisinger C (2012) Blood pressure and lipid management fall far short in persons with type 2 diabetes: results from the DIAB-CORE Consortium including six German population-based studies. Cardiovasc Diabetol 11:50. https://doi.org/10.1186/1475-2840-11-50
Ruggenenti P, Abbate M, Ruggiero B, Rota S, Trillini M, Aparicio C, Parvanova A, Petrov Iliev I, Pisanu G, Perna A, Russo A, Diadei O, Martinetti D, Cannata A, Carrara F, Ferrari S, Stucchi N, Remuzzi G, Fontana L, C.RE.S.O. Study Group (2017) Renal and systemic effects of calorie restriction in patients with type 2 diabetes with abdominal obesity: a randomized controlled trial. Diabetes. 66(1):75–86. https://doi.org/10.2337/db16-0607
Rysz J, Franczyk B, Ławiński J, Gluba-Brzózka A (2020) Oxidative stress in ESRD patients on dialysis and the risk of cardiovascular diseases. Antioxidants (Basel) 9(11):1079. https://doi.org/10.3390/antiox9111079
Sabater-Lleal M, Huang J, Chasman D, Naitza S, Dehghan A, Johnson AD, Teumer A, Reiner AP, Folkersen L, Basu S, Rudnicka AR, Trompet S, Mälarstig A, Baumert J, Bis JC, Guo X, Hottenga JJ, Shin SY, Lopez LM, Lahti J, Tanaka T, Yanek LR, Oudot-Mellakh T, Wilson JF, Navarro P, Huffman JE, Zemunik T, Redline S, Mehra R, Pulanic D, Rudan I, Wright AF, Kolcic I, Polasek O, Wild SH, Campbell H, Curb JD, Wallace R, Liu S, Eaton CB, Becker DM, Becker LC, Bandinelli S, Räikkönen K, Widen E, Palotie A, Fornage M, Green D, Gross M, Davies G, Harris SE, Liewald DC, Starr JM, Williams FM, Grant PJ, Spector TD, Strawbridge RJ, Silveira A, Sennblad B, Rivadeneira F, Uitterlinden AG, Franco OH, Hofman A, van Dongen J, Willemsen G, Boomsma DI, Yao J, Swords Jenny N, Haritunians T, McKnight B, Lumley T, Taylor KD, Rotter JI, Psaty BM, Peters A, Gieger C, Illig T, Grotevendt A, Homuth G, Völzke H, Kocher T, Goel A, Franzosi MG, Seedorf U, Clarke R, Steri M, Tarasov KV, Sanna S, Schlessinger D, Stott DJ, Sattar N, Buckley BM, Rumley A, Lowe GD, McArdle WL, Chen MH, Tofler GH, Song J, Boerwinkle E, Folsom AR, Rose LM, Franco-Cereceda A, Teichert M, Ikram MA, Mosley TH, Bevan S, Dichgans M, Rothwell PM, Sudlow CL, Hopewell JC, Chambers JC, Saleheen D, Kooner JS, Danesh J, Nelson CP, Erdmann J, Reilly MP, Kathiresan S, Schunkert H, Morange PE, Ferrucci L, Eriksson JG, Jacobs D, Deary IJ, Soranzo N, Witteman JC, de Geus EJ, Tracy RP, Hayward C, Koenig W, Cucca F, Jukema JW, Eriksson P, Seshadri S, Markus HS, Watkins H, Samani NJ, VTE Consortium; STROKE Consortium; Wellcome Trust Case Control Consortium 2 (WTCCC2); C4D Consortium; CARDIoGRAM Consortium, Wallaschofski H, Smith NL, Tregouet D, Ridker PM, Tang W, Strachan DP, Hamsten A, O’Donnell CJ (2013) Multiethnic meta-analysis of genome-wide association studies in >100 000 subjects identifies 23 fibrinogen-associated Loci but no strong evidence of a causal association between circulating fibrinogen and cardiovascular disease. Circulation 128(12):1310–1324. https://doi.org/10.1161/CIRCULATIONAHA.113.002251
Sakai C, Ishida M, Ohba H, Yamashita H, Uchida H, Yoshizumi M, Ishida T (2017) Fish oil omega-3 polyunsaturated fatty acids attenuate oxidative stress-induced DNA damage in vascular endothelial cells. PLoS One 12(11):e0187934. https://doi.org/10.1371/journal.pone.0187934
Samaranayaka AG, Li-Chan EC (2011) Food-derived peptidic antioxidants: a review of their production, assessment, and potential applications. J Funct Foods 3:229–254. https://doi.org/10.1016/j.jff.2011.05.006
Samaras K, Botelho NK, Chisholm DJ, Lord RV (2010) Subcutaneous and visceral adipose tissue gene expression of serum adipokines that predict type 2 diabetes. Obesity (Silver Spring) 18(5):884–889. https://doi.org/10.1038/oby.2009.443
Sampieri CL, Orozco-Ortega RA (2018) Matrix metalloproteinases and tissue inhibitors of metalloproteinases in chronic kidney disease and acute kidney injury: a systematic review of the literature. Hippokratia. 22(3):99–104
Samuni Y, Goldstein S, Dean OM, Berk M (2013) The chemistry and biological activities of N-acetylcysteine. Biochim Biophys Acta 1830(8):4117–4129. https://doi.org/10.1016/j.bbagen.2013.04.016
Santillo M, Colantuoni A, Mondola P, Guida B, Damiano S (2015) NOX signaling in molecular cardiovascular mechanisms involved in the blood pressure homeostasis. Front Physiol 6:194. https://doi.org/10.3389/fphys.2015.00194
Sárközy M, Kovács ZZA, Kovács MG, Gáspár R, Szűcs G, Dux L (2018) Mechanisms and modulation of oxidative/nitrative stress in type 4 cardio-renal syndrome and renal sarcopenia. Front Physiol 9:1648. https://doi.org/10.3389/fphys.2018.01648
Sasser JM, Cunningham MW Jr, Baylis C (2014) Serelaxin reduces oxidative stress and asymmetric dimethylarginine in angiotensin II-induced hypertension. Am J Phys Renal Phys 307(12):F1355–F1362. https://doi.org/10.1152/ajprenal.00407.2014
Scheepers LE, Wei FF, Stolarz-Skrzypek K, Malyutina S, Tikhonoff V, Thijs L, Salvi E, Barlassina C, Filipovský J, Casiglia E, Nikitin Y, Kawecka-Jaszcz K, Manunta P, Cusi D, Boonen A, Staessen JA, Arts IC (2016) Xanthine oxidase gene variants and their association with blood pressure and incident hypertension: a population study. J Hypertens 34(11):2147–2154. https://doi.org/10.1097/HJH.0000000000001077
Seals DR, Kaplon RE, Gioscia-Ryan RA, LaRocca TJ (2014) You're only as old as your arteries: translational strategies for preserving vascular endothelial function with aging. Physiology (Bethesda) 29(4):250–264. https://doi.org/10.1152/physiol.00059.2013
Semple DJ, Bhandari S, Seymour AM (2012) Uremic cardiomyopathy is characterized by loss of the cardioprotective effects of insulin. Am J Phys Renal Phys 303(9):F1275–F1286. https://doi.org/10.1152/ajprenal.00048.2012
Sena LA, Chandel NS (2012) Physiological roles of mitochondrial reactive oxygen species. Mol Cell 48(2):158–167. https://doi.org/10.1016/j.molcel.2012.09.025
Sena CM, Pereira AM, Seiça R (2013) Endothelial dysfunction — a major mediator of diabetic vascular disease. Biochim Biophys Acta 1832(12):2216–2231. https://doi.org/10.1016/j.bbadis.2013.08.006
Senoner T, Dichtl W (2019) Oxidative stress in cardiovascular diseases: still a therapeutic target? Nutrients. 11(9):2090. https://doi.org/10.3390/nu11092090
Shadel GS, Horvath TL (2015) Mitochondrial ROS signaling in organismal homeostasis. Cell. 163(3):560–569. https://doi.org/10.1016/j.cell.2015.10.001
Sheehan AL, Carrell S, Johnson B, Stanic B, Banfi B, Miller FJ Jr (2011) Role for Nox1 NADPH oxidase in atherosclerosis. Atherosclerosis. 216(2):321–326. https://doi.org/10.1016/j.atherosclerosis.2011.02.028
Shin SJ, Chung S, Kim SJ, Lee EM, Yoo YH, Kim JW, Ahn YB, Kim ES, Moon SD, Kim MJ, Ko SH (2016) Effect of sodium-glucose co-transporter 2 inhibitor, Dapagliflozin, on renal renin-angiotensin system in an animal model of type 2 diabetes. PLoS One 11(11):e0165703. https://doi.org/10.1371/journal.pone.0165703
Shinmura K, Tamaki K, Sano M, Nakashima-Kamimura N, Wolf AM, Amo T, Ohta S, Katsumata Y, Fukuda K, Ishiwata K, Suematsu M, Adachi T (2011) Caloric restriction primes mitochondria for ischemic stress by deacetylating specific mitochondrial proteins of the electron transport chain. Circ Res 109(4):396–406. https://doi.org/10.1161/CIRCRESAHA.111.243097
Shirakawa R, Yokota T, Nakajima T, Takada S, Yamane M, Furihata T, Maekawa S, Nambu H, Katayama T, Fukushima A, Saito A, Ishimori N, Dela F, Kinugawa S, Anzai T (2019) Mitochondrial reactive oxygen species generation in blood cells is associated with disease severity and exercise intolerance in heart failure patients. Sci Rep 9(1):14709. https://doi.org/10.1038/s41598-019-51298-3
Sies H, Berndt C, Jones DP (2017) Oxidative stress. Annu Rev Biochem 86:715–748. https://doi.org/10.1146/annurev-biochem-061516-045037
Silva-Cutini MA, Almeida SA, Nascimento AM, Abreu GR, Bissoli NS, Lenz D, Endringer DC, Brasil GA, Lima EM, Biancardi VC, Andrade TU (2019) Long-term treatment with kefir probiotics ameliorates cardiac function in spontaneously hypertensive rats. J Nutr Biochem 66:79–85. https://doi.org/10.1016/j.jnutbio.2019.01.006
Singh CK, Chhabra G, Ndiaye MA, Garcia-Peterson LM, Mack NJ, Ahmad N (2017) The role of sirtuins in antioxidant and redox signaling. Antioxid Redox Signal 28(8):643–661. https://doi.org/10.1089/ars.2017.7290
Six I, Flissi N, Lenglet G, Louvet L, Kamel S, Gallet M, Massy ZA, Liabeuf S (2020) Uremic toxins and vascular dysfunction. Toxins (Basel) 12(6):404. https://doi.org/10.3390/toxins12060404
Sokoła-Wysoczańska E, Wysoczański T, Wagner J, Czyż K, Bodkowski R, Lochyński S, Patkowska-Sokoła B (2018) Polyunsaturated fatty acids and their potential therapeutic role in cardiovascular system disorders—a review. Nutrients. 10(10):1561. https://doi.org/10.3390/nu10101561
Soler-Botija C, Gálvez-Montón C, Bayés-Genís A (2019) Epigenetic biomarkers in cardiovascular diseases. Front Genet 10:950. https://doi.org/10.3389/fgene.2019.00950
Soufi N, Hall AM, Chen Z, Yoshino J, Collier SL, Mathews JC, Brunt EM, Albert CJ, Graham MJ, Ford DA, Finck BN (2014) Inhibiting monoacylglycerol acyltransferase 1 ameliorates hepatic metabolic abnormalities but not inflammation and injury in mice. J Biol Chem 289(43):30177–30188. https://doi.org/10.1074/jbc.M114.595850
Speakman JR, Mitchell SE (2011) Caloric restriction. Mol Asp Med 32(3):159–221. https://doi.org/10.1016/j.mam.2011.07.001
Stasch JP, Pacher P, Evgenov OV (2011) Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation. 123(20):2263–2273. https://doi.org/10.1161/CIRCULATIONAHA.110.981738
Steinbacher P, Eckl P (2015) Impact of oxidative stress on exercising skeletal muscle. Biomolecules 5(2):356–377. https://doi.org/10.3390/biom5020356
Steven S, Frenis K, Oelze M, Kalinovic S, Kuntic M, Bayo Jimenez MT, Vujacic-Mirski K, Helmstädter J, Kröller-Schön S, Münzel T, Daiber A (2019) Vascular inflammation and oxidative stress: major triggers for cardiovascular disease. Oxidative Med Cell Longev 2019:7092151. https://doi.org/10.1155/2019/7092151
Stock NS, Bain G, Zunic J, Li Y, Ziff J, Roppe J, Santini A, Darlington J, Prodanovich P, King CD, Baccei C, Lee C, Rong H, Chapman C, Broadhead A, Lorrain D, Correa L, Hutchinson JH, Evans JF, Prasit P (2011) 5-Lipoxygenase-activating protein (FLAP) inhibitors. Part 4: development of 3-[3-tert-butylsulfanyl-1-[4-(6-ethoxypyridin-3-yl)benzyl]-5-(5-methylpyridin-2-ylmethoxy)-1H-indol-2-yl]-2,2-dimethylpropionic acid (AM803), a potent, oral, once daily FLAP inhibitor. J Med Chem 54(23):8013–8029. https://doi.org/10.1021/jm2008369
Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, Mehran R, McPherson J, Farhat N, Marso SP, Parise H, Templin B, White R, Zhang Z, Serruys PW, Investigators PROSPECT (2011) A prospective natural-history study of coronary atherosclerosis. N Engl J Med 364(3):226–235. https://doi.org/10.1056/NEJMoa1002358
Straub AC, Lohman AW, Billaud M, Johnstone SR, Dwyer ST, Lee MY, Bortz PS, Best AK, Columbus L, Gaston B, Isakson BE (2012) Endothelial cell expression of haemoglobin α regulates nitric oxide signalling. Nature. 491(7424):473–477. https://doi.org/10.1038/nature11626
Subbiah AK, Chhabra YK, Mahajan S (2016) Cardiovascular disease in patients with chronic kidney disease: a neglected subgroup. Heart Asia 8(2):56–61. https://doi.org/10.1136/heartasia-2016-010809
Sugamura K, Keaney JF Jr (2011) Reactive oxygen species in cardiovascular disease. Free Radic Biol Med 51(5):978–992. https://doi.org/10.1016/j.freeradbiomed.2011.05.004
Sugizaki T, Zhu S, Guo G, Matsumoto A, Zhao J, Endo M, Horiguchi H, Morinaga J, Tian Z, Kadomatsu T, Miyata K, Itoh H, Oike Y (2017) Treatment of diabetic mice with the SGLT2 inhibitor TA-1887 antagonizes diabetic cachexia and decreases mortality. NPJ Aging Mech Dis 3:12. https://doi.org/10.1038/s41514-017-0012-0
Szeto HH (2014) First-in-class cardiolipin-protective compound as a therapeutic agent to restore mitochondrial bioenergetics. Br J Pharmacol 171(8):2029–2050. https://doi.org/10.1111/bph.12461
Tahara A, Kurosaki E, Yokono M, Yamajuku D, Kihara R, Hayashizaki Y, Takasu T, Imamura M, Li Q, Tomiyama H, Kobayashi Y, Noda A, Sasamata M, Shibasaki M (2014) Effects of sodium-glucose cotransporter 2 selective inhibitor ipragliflozin on hyperglycaemia, oxidative stress, inflammation and liver injury in streptozotocin-induced type 1 diabetic rats. J Pharm Pharmacol 66(7):975–987. https://doi.org/10.1111/jphp.12223
Talhout R, Schulz T, Florek E, van Benthem J, Wester P, Opperhuizen A (2011) Hazardous compounds in tobacco smoke. Int J Environ Res Public Health 8(2):613–628. https://doi.org/10.3390/ijerph8020613
Tan Z, Ouyang D, Chen Y, Zhou G, Cao S, Wang Y, Peng X, Zhou H (2010) Development and validation of a LC-MS/MS method for the determination of clebopride and its application to a pharmacokinetics study in healthy Chinese volunteers. J Chromatogr B Anal Technol Biomed Life Sci 878(23):2072–2076. https://doi.org/10.1016/j.jchromb.2010.06.006
Tang GY, Meng X, Li Y, Zhao CN, Liu Q, Li HB (2017) Effects of vegetables on cardiovascular diseases and related mechanisms. Nutrients. 9(8):857. https://doi.org/10.3390/nu9080857
Taylor D, Bhandari S, Seymour AM (2015) Mitochondrial dysfunction in uremic cardiomyopathy. Am J Phys Renal Phys 308(6):F579–F587. https://doi.org/10.1152/ajprenal.00442.2014
Teixeira BC, Lopes AL, Macedo RCO, Correa CS, Ramis TR, Ribeiro JL, Reischak-Oliveira A (2014) Inflammatory markers, endothelial function and cardiovascular risk. J Vasc Bras 13:108–115. https://doi.org/10.1590/jvb.2014.054
Terami N, Ogawa D, Tachibana H, Hatanaka T, Wada J, Nakatsuka A, Eguchi J, Horiguchi CS, Nishii N, Yamada H, Takei K, Makino H (2014) Long-term treatment with the sodium glucose cotransporter 2 inhibitor, dapagliflozin, ameliorates glucose homeostasis and diabetic nephropathy in db/db mice. PLoS One 9(6):e100777. https://doi.org/10.1371/journal.pone.0100777
Todur SP, Ashavaid TF (2012) Association of Sp1 tandem repeat polymorphism of ALOX5 with coronary artery disease in Indian subjects. Clin Transl Sci 5(5):408–411. https://doi.org/10.1111/j.1752-8062.2011.00396.x
Tousoulis D, Hatzis G, Papageorgiou N, Androulakis E, Bouras G, Giolis A, Bakogiannis C, Siasos G, Latsios G, Antoniades C, Stefanadis C (2012) Assessment of acute coronary syndromes: focus on novel biomarkers. Curr Med Chem 19(16):2572–2587. https://doi.org/10.2174/092986712800493011
Touyz RM, Briones AM (2011) Reactive oxygen species and vascular biology: implications in human hypertension. Hypertens Res 34(1):5–14. https://doi.org/10.1038/hr.2010.201
Turell L, Radi R, Alvarez B (2013) The thiol pool in human plasma: the central contribution of albumin to redox processes. Free Radic Biol Med 65:244–253. https://doi.org/10.1016/j.freeradbiomed.2013.05.050
Uddin MS, Mamun AA, Jakaria M, Thangapandiyan S, Ahmad J, Rahman MA, Mathew B, Abdel-Daim MM, Aleya L (2020) Emerging promise of sulforaphane-mediated Nrf2 signaling cascade against neurological disorders. Sci Total Environ 707:135624. https://doi.org/10.1016/j.scitotenv.2019.135624
Vardi M, Levy NS, Levy AP (2013) Vitamin E in the prevention of cardiovascular disease: the importance of proper patient selection. J Lipid Res 54(9):2307–2314. https://doi.org/10.1194/jlr.R026641
Varga ZV, Kupai K, Szűcs G, Gáspár R, Pálóczi J, Faragó N, Zvara A, Puskás LG, Rázga Z, Tiszlavicz L, Bencsik P, Görbe A, Csonka C, Ferdinandy P, Csont T (2013) MicroRNA-25-dependent up-regulation of NADPH oxidase 4 (NOX4) mediates hypercholesterolemia-induced oxidative/nitrative stress and subsequent dysfunction in the heart. J Mol Cell Cardiol 62:111–121. https://doi.org/10.1016/j.yjmcc.2013.05.009
Vasquez EC, Pereira TMC, Peotta VA, Baldo MP, Campos-Toimil M (2019) Probiotics as beneficial dietary supplements to prevent and treat cardiovascular diseases: uncovering their impact on oxidative stress. Oxidative Med Cell Longev 2019:3086270. https://doi.org/10.1155/2019/3086270
Veskoukis AS, Tsatsakis AM, Kouretas D (2012) Dietary oxidative stress and antioxidant defense with an emphasis on plant extract administration. Cell Stress Chaperones 17(1):11–21. https://doi.org/10.1007/s12192-011-0293-3
Victor VM, Rocha M, Herance R, Hernandez-Mijares A (2011) Oxidative stress and mitochondrial dysfunction in type 2 diabetes. Curr Pharm Des 17(36):3947–3958. https://doi.org/10.2174/138161211798764915
Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Südhof TC, Wernig M (2010) Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 463(7284):1035–1041. https://doi.org/10.1038/nature08797
Villareal DT, Aguirre L, Gurney AB, Waters DL, Sinacore DR, Colombo E, Armamento-Villareal R, Qualls C (2017) Aerobic or resistance exercise, or both, in dieting obese older adults. N Engl J Med 376(20):1943–1955. https://doi.org/10.1056/NEJMoa1616338
Vinci MC, Polvani G, Pesce M (2013) Epigenetic programming and risk: the birthplace of cardiovascular disease? Stem Cell Rev Rep 9(3):241–253. https://doi.org/10.1007/s12015-012-9398-z
Vinciguerra M, Santini MP, Martinez C, Pazienza V, Claycomb WC, Giuliani A, Rosenthal N (2012) mIGF-1/JNK1/SirT1 signaling confers protection against oxidative stress in the heart. Aging Cell 11(1):139–149. https://doi.org/10.1111/j.1474-9726.2011.00766.x
Wallace TC, Slavin M, Frankenfeld CL (2016) Systematic review of anthocyanins and markers of cardiovascular disease. Nutrients. 8(1):32. https://doi.org/10.3390/nu8010032
Wang RS, Oldham WM, Maron BA, Loscalzo J (2018a) Systems biology approaches to redox metabolism in stress and disease states. Antioxid Redox Signal 29(10):953–972. https://doi.org/10.1089/ars.2017.7256
Wang X, Li W, Song F, Wang L, Fu Q, Cao S, Gan Y, Zhang W, Yue W, Yan F, Shi W, Wang X, Zhang H, Zhang H, Wang Z, Lu Z (2018b) Carotid atherosclerosis detected by ultrasonography: a national cross-sectional study. J Am Heart Assoc 7(8):e008701. https://doi.org/10.1161/JAHA.118.008701
Welsh P, Grassia G, Botha S, Sattar N, Maffia P (2017) Targeting inflammation to reduce cardiovascular disease risk: a realistic clinical prospect? Br J Pharmacol 174(22):3898–3913. https://doi.org/10.1111/bph.13818
White WB, Saag KG, Becker MA, Borer JS, Gorelick PB, Whelton A, Hunt B, Castillo M, Gunawardhana L, CARES Investigators (2018) Cardiovascular safety of febuxostat or allopurinol in patients with gout. N Engl J Med 378(13):1200–1210. https://doi.org/10.1056/NEJMoa1710895
Wiciński M, Górski K, Wódkiewicz E, Walczak M, Nowaczewska M, Malinowski B (2020) Vasculoprotective effects of vildagliptin. Focus on Atherogenesis. Int J Mol Sci 21(7):2275. https://doi.org/10.3390/ijms21072275
Wickremasinghe D, Peiris H, Chandrasena LG, Senaratne V, Perera R (2016) Case control feasibility study assessing the association between severity of coronary artery disease with glutathione peroxidase-1 (GPX-1) and GPX-1 polymorphism (Pro198Leu). BMC Cardiovasc Disord 16:111. https://doi.org/10.1186/s12872-016-0280-9
Widmer RJ, Flammer AJ, Lerman LO, Lerman A (2015) The Mediterranean diet, its components, and cardiovascular disease. Am J Med 128(3):229–238. https://doi.org/10.1016/j.amjmed.2014.10.014
Xu XF, Ma XL, Shen Z, Wu XL, Cheng F, Du LZ (2010) Epigenetic regulation of the endothelial nitric oxide synthase gene in persistent pulmonary hypertension of the newborn rat. J Hypertens 28(11):2227–2235. https://doi.org/10.1097/HJH.0b013e32833e08f1
Yamaguchi M, Fukasawa S (2021) Is inflammation a friend or foe for orthodontic treatment?: inflammation in orthodontically induced inflammatory root resorption and accelerating tooth movement. Int J Mol Sci 22(5):2388. https://doi.org/10.3390/ijms22052388
Yamamoto T, Tamaki K, Shirakawa K, Ito K, Yan X, Katsumata Y, Anzai A, Matsuhashi T, Endo J, Inaba T, Tsubota K, Sano M, Fukuda K, Shinmura K (2016) Cardiac Sirt1 mediates the cardioprotective effect of caloric restriction by suppressing local complement system activation after ischemia-reperfusion. Am J Physiol Heart Circ Physiol 310(8):H1003–H1014. https://doi.org/10.1152/ajpheart.00676.2015
Yang CS, Ho CT, Zhang J, Wan X, Zhang K, Lim J (2018a) Antioxidants: differing meanings in food science and health science. J Agric Food Chem 66(12):3063–3068. https://doi.org/10.1021/acs.jafc.7b05830
Yang J, Yin HS, Cao YJ, Jiang ZA, Li YJ, Song MC, Wang YF, Wang ZH, Yang R, Jiang YF, Sun JP, Liu BY, Wang C (2018b) Arctigenin attenuates ischemia/reperfusion induced ventricular arrhythmias by decreasing oxidative stress in rats. Cell Physiol Biochem 49(2):728–742. https://doi.org/10.1159/000493038
Yaribeygi H, Atkin SL, Butler AE, Sahebkar A (2019) Sodium-glucose cotransporter inhibitors and oxidative stress: an update. J Cell Physiol 234(4):3231–3237. https://doi.org/10.1002/jcp.26760
Yin H, Xu L, Porter NA (2011) Free radical lipid peroxidation: mechanisms and analysis. Chem Rev 111(10):5944–5972. https://doi.org/10.1021/cr200084z
Yuyun MF, Ng LL, Ng GA (2018) Endothelial dysfunction, endothelial nitric oxide bioavailability, tetrahydrobiopterin, and 5-methyltetrahydrofolate in cardiovascular disease. Where are we with therapy? Microvasc Res 119:7–12. https://doi.org/10.1016/j.mvr.2018.03.012
Zeng Z, Jin Y, Zheng Y, Xiang L, Yang J, Peng L, Wei Q, Yang M, Cheng Q, Rong X, Piao S, Guo J (2021) Interaction between overweight, obesity and smoking on the risk of pre-diabetes and type 2 diabetes in Guangdong, China. https://doi.org/10.21203/rs.3.rs-301611/v1
Zhang QJ, Chen HZ, Wang L, Liu DP, Hill JA, Liu ZP (2011) The histone trimethyllysine demethylase JMJD2A promotes cardiac hypertrophy in response to hypertrophic stimuli in mice. J Clin Invest 121(6):2447–2456. https://doi.org/10.1172/JCI46277
Zhao Z (2018) Correlation analysis of urine proteins and inflammatory cytokines with osteoporosis in patients with diabetic nephropathy. J Musculoskelet Neuronal Interact 18(3):348–353
Zhao J, Wang F, Zhang Y, Jiao L, Lau WB, Wang L, Liu B, Gao E, Koch WJ, Ma XL, Wang Y (2013) Sevoflurane preconditioning attenuates myocardial ischemia/reperfusion injury via caveolin-3-dependent cyclooxygenase-2 inhibition. Circulation. 128(11 Suppl 1):S121–S129. https://doi.org/10.1161/CIRCULATIONAHA.112.000045
Zhu Y, Ling W, Guo H, Song F, Ye Q, Zou T, Li D, Zhang Y, Li G, Xiao Y, Liu F, Li Z, Shi Z, Yang Y (2013) Anti-inflammatory effect of purified dietary anthocyanin in adults with hypercholesterolemia: a randomized controlled trial. Nutr Metab Cardiovasc Dis 23(9):843–849. https://doi.org/10.1016/j.numecd.2012.06.005
Zhu N, Huang B, Jiang W (2021) Targets of vitamin C with therapeutic potential for cardiovascular disease and underlying mechanisms: a study of network pharmacology. Front Pharmacol 11:591337. https://doi.org/10.3389/fphar.2020.591337
Zielonka J, Joseph J, Sikora A, Hardy M, Ouari O, Vasquez-Vivar J, Cheng G, Lopez M, Kalyanaraman B (2017) Mitochondria-targeted triphenylphosphonium-based compounds: syntheses, mechanisms of action, and therapeutic and diagnostic applications. Chem Rev 117(15):10043–10120. https://doi.org/10.1021/acs.chemrev.7b00042
Acknowledgements
Deep thanks from authors to the editor of Environmental Science and Pollution Research Journal and reviewers who improve this manuscript to appear in this good form.
Author information
Authors and Affiliations
Contributions
All authors AK and TD are equally contributed to writing the current review and design all the figures as well the final editing and formatting and final revision. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Donia, T., Khamis, A. Management of oxidative stress and inflammation in cardiovascular diseases: mechanisms and challenges. Environ Sci Pollut Res 28, 34121–34153 (2021). https://doi.org/10.1007/s11356-021-14109-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-14109-9