Abstract
In the current study, sweet potato peel (Ipomoea batatas) was observed as the most favorable substrate for the maximum synthesis of α-1,4-glucosidase among various agro-industrial residues. Bacillus licheniformis KIBGE-IB4 produced 6533.0 U ml−1 of α-1,4-glucosidase when growth medium was supplemented with 1% dried and crushed sweet potato peel. It was evident from the results that bacterial isolate secreted 6539.0 U ml−1 of α-1,4-glucosidase in the presence of 0.4% peptone and meat extract with 0.1% yeast extract. B. licheniformis KIBGE-IB4 released 6739.0 and 7190.0 U ml−1 of enzyme at 40 °C and pH 7.0, respectively. An improved and cost-effective growth medium design resulted 8590.0 U ml−1 of α-1,4-glucosidase with 1.3-fold increase as compared to initial amount from B. licheniformis KIBGE-IB4. This enzyme can be used to fulfill the accelerating demand of food and pharmaceutical industries. Further purification and immobilization of this enzyme can also enhance its utility for various commercial applications.

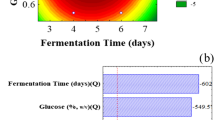
Pictorial representation of maltase production from sweet potato peel






Similar content being viewed by others
References
Adhyaru DN, Bhatt NS, Modi HA (2014) Enhanced production of cellulase-free, thermo-alkali-solvent-stable xylanase from Bacillus altitudinis DHN8, its characterization and application in sorghum straw saccharification. Biocatal Agr Biotechnol 3:182–190
Adrio JL, Demain AL (2014) Microbial enzymes: tools for biotechnological processes. Biomolecules 4:117–139
Ahmed I, Zia MA, Hussain MA, Akram Z, Naveed MT, Nowrouzi A (2015) Bioprocessing of citrus waste peel for induced pectinase production by Aspergillus niger; its purification and characterization. J Radiat Res Appl Sci. doi:10.1016/j.jrras.2015.11.003
Amin M, Bhatti HN, Zuber M, Bhatti IA, Asgher M (2014) Potential use of agricultural wastes for the production of lipase by Aspergillus melleus under solid state fermentation. J Anim Plant Sci 23:1430–1437
Anwar Z, Gulfraz M, Irshad M (2014) Agro-industrial lignocellulosic biomass a key to unlock the future bio-energy: a brief review. J Radiat Res Appl Sci 7:163–173
Arnold FH, Wintrode PL, Miyazaki K, Gershenson A (2001) How enzymes adapt: lessons from directed evolution. Trends Biochem Sci 26:100–106
Banerjee R, De KB, Bhattacharyya BC (1992) Optimization of extracellular protease biosynthesis by newly isolated Rhizopus oryzae. Indian J Technol 30:275–280
Betiku E, Akindolani OO, Ismaila AR (2013) Enzymatic hydrolysis optimization of sweet potato (Ipomoea batatas) peel using a statistical approach. Braz J Chem Eng 30:467–476
Bhargav S, Panda P, Ali M, Javad S (2008) Solid-state fermentation: an overview. Chem Biochem Eng Q 22:49–70
Bibi Z, Ansari A, Zohra RR, Aman A, Ul Qader SA (2014) Production of xylan degrading endo-1,4-β-xylanase from thermophilic Geobacillus stearothermophilus KIBGE-IB29. J Radiat Res Appl Sci 7:478–485
Broiheir K (2006) Sweet potato: tubers delivers top-notch nutrition. Environ Nutr 29:8–9
Czuchajowska Z, Szczodrak J, Pomeranz Y (1992) Characterization and estimation of barley polysaccharides by near-infrared spectroscopy. 1. Barleys, starches and β-D-glucans. Cereal Chem 69:413–418
Deb P, Talukdar SA, Mohsinal K, Sarker PK, Sayem SMA (2013) Production and partial characterization of extracellular amylase enzyme from Bacillus amyloliquefaciens P-001. Springer Plus 2:154. doi:10.1186/2193-1801-2-154
Djekrif-Dakhmouche S, Gheribi-Aoulmi Z, Meraihi Z, Bennamoun L (2005) Application of a statistical design to the optimization of culture medium for α-amylase production by Aspergillus niger ATCC 16404 grown on orange waste powder. J Food Eng 73:190–197
Dos Santos TC, Gomes DPP, Bonomo RCF, Franco M (2012) Optimisation of solid state fermentation of potato peel for the production of cellulolytic enzymes. Food Chem 133:1299–1304
Dos Santos TC, Filho GA, Oliveira AC, Rocha TJO, Machado FPP, Bonomo RCF, Mota KIA, Franco M (2013) Application of response surface methodology for producing cellulolytic enzymes by solid-state fermentation from the purple mombin (Spondias purpurea L.) residue. Food Sci Biotechnol 22:1–7. doi:10.1007/s10068-013-0001-4
Durrani Y, Wahab S, Javid-Ullah MAC (2002) Feutrell’s early waste utilization in by-product development and its utilization in bakery products. Sarhad J Agric 18:463–466
El-Hadi A, El-Nour SA, Hammad A, Kamel Z, Anwar M (2014) Optimization of cultural and nutritional conditions for carboxymethylcellulase production by Aspergillus hortai. J Radiat Res Appl Sci 7:23–28
El-Shishtawy RM, Mohamed SA, Asiri AM, Gomaa AM, Ibrahim IH, Al-Talhi HA (2014) Solid fermentation of wheat bran for hydrolytic enzymes production and saccharification content by a local isolate Bacillus megaterium. BMC Biotechnol 14:29. doi:10.1186/1472-6750-14-29
Garciaa NFL, Santosa FR, Gonçalvesb FA, Paza MF, Fonsecab GG, Leitea RSR (2015) Production of β-glucosidase on solid-state fermentation by Lichtheimia ramosa in agroindustrial residues: characterization and catalytic properties of the enzymatic extract. Electron J Biotechnol 18:314–319
Gomes J, Purkarthofer H, Hayn M, Kapplmüller J, Sinner M, Steiner W (1993) Production of a high level of cellulase-free xylanase by the thermophilic fungus Thermomyces lanuginosus in laboratory and pilot scales using lignocellulosic materials. Appl Microbiol Biotechnol 39:700–707
Gupta R, Gigras P, Mohapatra H, Goswami VK, Chauhan B (2003) Microbial α-amylases: a biotechnological prospective. Process Biochem 38:1599–1616
Gyawali R, Ibrahim SA (2012) Impact of plant derivatives on the growth of foodborne pathogens and the functionality of probiotics. Appl Microb Biotechnol 95:29–45
Hassid WZ, Abraham S (1957) Methods in enzymology III. Academic, New York, pp. 34–35
Hull P (2010) Glucose syrups: technology and applications. Wiley-Blackwell, Chichester
Ibrahim ASS, Al-Salamah AA, Elbadawi YB, El-Tayeb MA, Ibrahim SSS (2015) Production of extracellular alkaline protease by new halotolerant alkaliphilic Bacillus sp. NPST-AK15 isolated from hyper saline soda lakes. Electron J Biotechnol 18:236–243
Irfan M, Nadeem M, Syed Q (2014) One-factor-at-a-time (OFAT) optimization of xylanase production from Trichoderma viride-IR05 in solid-state fermentation. J Radiat Res Appl Sci 7:317–326
Johnson R, Padmaja G, Moorthy SN (2009) Comparative production of glucose and high fructose syrup from cassava and sweet potato roots by direct conversion techniques. Innov Food Sci Emerg Technol 10:616–620
Julia BM, Belén AM, Georgina B, Beatriz F (2016) Potential use of soybean hulls and waste paper as supports in SSF for cellulase production by Aspergillus niger. Bio Biocatal Agric Biotechnol 6:1–8
Juturu V, Wu JC (2014) Microbial cellulases: engineering, production and applications. Renew Sust Energ Rev 33:188–203
Kamran A, Bibi Z, Aman A, Ul Qader SA (2016) Lactose hydrolysis approach: isolation and production of β-galactosidase from newly isolated Bacillus strain B-2. Biocatal Agric Biotechnol 5:99–103
Karim A, Nawaz MA, Aman A, Ul Qader SA (2015) Hyper production of cellulose degrading endo (1,4) β-D-glucanase from Bacillus licheniformis KIBGE-IB2. J Radiat Res Appl Sci 8:160–165
Kathiresan K, Manivannan S (2006) α-Amylase production by Penicillium fellutanum isolated from mangrove rhizospheric soil. Afr J Biotechnol 5:829–832
Li L, Zhang L, Li K, Wang Y, Gao C, Han B, Ma C, Xu P (2013) A newly isolated Bacillus licheniformis strain thermophilically produces 2,3-butanediol, a platform and fuel bio-chemical. Biotechnol Biofuels 6:123. doi:10.1186/1754-6834-6-123
Lorenz P, Schleper C (2002) Metagenome—a challenging source of enzyme discovery. J Mol Catal B Enzyme 19:13–19
Maiti S, Sarma SJ, Brar SK, Bihan YL, Drogui P, Buelna G, Verma M (2016) Agro-industrial wastes as feedstock for sustainable bio-production of butanol by Clostridium beijerinckii. Food Bioprod Process. doi:10.1016/j.fbp.2016.01.002
Mfombep PM, Senwosoil ZN (2012) Soil maltase activity by glucose-oxidase peroxidase system. 3. Biotech 2:225–231
Mohamed RA, Salleh AB, Rahman RNZRA, Basri M, Leow TC (2012) Isolation of the encoding gene for a thermostable α-glucosidase from Geobacillus stearothermophilus strain RM and its expression in Escherichia coli. Afr J Microbiol Res 6:2909–2917
Naseer N (2003) Analysing the effect of industrial waste on river Ravi (Pakistan). Pak J Appl Sci 3:587–603
Nawaz MA, Bibi Z, Aman A, Zohra RR, Ul Qader SA (2014) Enhanced production of maltase (α-glucosidase) from newly isolated strain of Bacillus licheniformis KIBGE-IB4. Pak J Pharm Sci 27:1437–1442
Neto WPF, Silvério HA, Dantas NO, Pasquini D (2013) Extraction and characterization of cellulose nanocrystals from agro-industrial residue—soy hulls. Ind Crop Prod 42:480–488
Padmaja G (2009) Uses and nutritional data of sweet potato. In: Loebenetein G, Thottappilly G (eds) The sweet potato. Springer, Netherlands, pp 189–234. doi:10.1007/978-1-4020-9475-0_11
Pandey A, Soccol CR, Nigam P, Brand D, Mohan R, Roussos S (2000) Biotechnological potential of coffee pulp and coffee husk for bioprocesses. Biochem Eng 6:153–162
Pant G, Prakash A, Pavani JVP, Bera S, Deviram GVNS, Kumar A, Panchpuri M, Prasuna RG (2015) Production, optimization and partial purification of protease from Bacillus subtilis. J Taibah Univ Sci 9:50–55
Pervez S, Siddiqui NN, Ansari A, Aman A, Qader SAU (2015) Phenotypic and molecular characterization of Aspergillus species for the production of starch-saccharifying amyloglucosidase. Ann Microbiol 65:2287–2291
Poturcu K, Ozmen I, Biyik HH (2016) Characterization of an alkaline thermostable pectin lyase from newly isolated Aspergillus niger _WHAK1 and its application on fruit juice clarification. Arab J Sci Eng. doi:10.1007/s13369-016-2041-6
Prakasham RS, Subba Rao C, Sreenivas Rao R, Sarma PN (2005) Alkaline protease production by an isolated Bacillus circulans under solid-state fermentation using agroindustrial waste: process parameters optimization. Biotechnol Prog 21:1380–1388
Prakasham RS, Subba Rao C, Sreenivas Rao R, Sarma PN (2007) Enhancement of acid amylase production by an isolated Aspergillus awamori. J Appl Microbiol 102:204–211
Rajoka MI, Akhtar MW, Hanif A, Khalid AL (2006) Production and characterization of a highly active cellobiase from Aspergillus niger grown in solid state fermentation. World J Microbiol Biotechnol 22:991–998
Rao CS, Sathish T, Laxmi MM, Laxmi GS, Rao RS, Prakasham RS (2008) Modeling and optimization of fermentation factors for enhancement of alkaline protease production by isolated Bacillus circulans using feed-forward neural network and genetic algorithm. J Appl Microbiol 104:889–898
Reddy SS, Krishnan C (2016) Production of xylooligosaccharides in SSF by Bacillus subtilis KCX006 producing β-xylosidase-free endo-xylanase and multiple xylan debranching enzymes. Prep Biochem Biotechnol 46:49–55
Rouches E, Herpoël-Gimbert I, Steyer JP, Carrere H (2016) Improvement of anaerobic degradation by white-rot fungi pretreatment of lignocellulosic biomass: a review. Renew Sust Energ Rev 59:179–198
Saha BC, Zeikus JG (1991) Characterization of thermostable α-glucosidase from Clostridium thermohydrosulfuricum 39E. Appl Microbiol Biotechnol 35:568–571
Schär-Zammaretti P, Dillmann M, D’Amico N, Affolter M, Ubbink J (2005) Influence of fermentation medium composition on physicochemical surface properties of Lactobacillus acidophilus. Appl Environ Microbiol 71:8165–8173
Seo J, Park TS, Kim JN, Ha JK, Seo S (2014) Production of endoglucanase, beta-glucosidase and xylanase by Bacillus licheniformis grown on minimal nutrient medium containing agriculture residues. Asian-Australas J Anim Sci 27:946–950
Shalini R, Gupta DK (2010) Utilization of pomace from apple processing industries: a review. J Food Sci Technol 47:365–371
Suganthi C, Mageswari A, Karthikeyan S, Anbalagan M, Sivakumar A, Gothandam KM (2013) Screening and optimization of protease production from a halotolerant Bacillus licheniformis isolated from saltern sediments. J Genet Eng Biotechnol 11:47–52
Swamy MV, Seenayya G (1996) Thermostable pullulanase and α-amylase activity from Clostridium thermosulfurogenes SV9-optimization of culture conditions for enzyme production. Process Biochem 31:157–162
Teague WM, Brumm PJ (1992) Commercial enzymes for starch hydrolysis products. In: Schenck FW, Hebeda RE (eds) Starch hydrolysis product: worldwide technology, production and application. VCH, New York, pp. 45–77
Trinder P (1969a) Determination of blood glucose using 4-aminophenazone as oxygen acceptor. J Clin Pathol 22:246
Trinder P (1969b) Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann Clin Biochem 6:24–27
Tye YY, Lee KT, Abdullah WNW, Leh CP (2016) The world availability of non-wood lignocellulosic biomass for the production of cellulosic ethanol and potential pretreatments for the enhancement of enzymatic saccharification. Renew Sust Energ Rev 60:155–172
Vandenbossche V, Brault J, Vilarem G, Hernández-Meléndez O, Vivaldo-Lima E, Hernández-Luna M, Barzana E, Duque A, Manzanares P, Ballesteros M, Mata J, Castellón E, Rigal L (2014) A new lignocellulosic biomass deconstruction process combining thermo-mechano chemical action and bio-catalytic enzymatic hydrolysis in a twin-screw extruder. Ind Crop Prod 55:258–266
Zhang J, Pan J, Guan G, Li Y, Xue W, Tang G, Wang A, Wang H (2008) Expression and high-yield production of extremely thermostable bacterial xylanase B in Aspergillus niger. Enzym Microb Technol 43:513–516
Zhang J, Tuomainen P, Siika-aho M, Viikari L (2011) Comparison of the synergistic action of two thermostable xylanases from GH families 10 and 11 with thermostable cellulases in lignocellulose hydrolysis. Bioresour Technol 102:9090–9095
Acknowledgements
This research work was financially supported by the Karachi Institute of Biotechnology and Genetic Engineering (KIBGE), University of Karachi, Karachi-75270, Pakistan.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Highlights
• Various agro-industrial wastes and pure substrates were evaluated as feedstock for α-1,4-glucosidase production.
• α-1,4-Glucosidase production was optimized using sweet potato peel as a sole carbon source.
• Incorporation of organic nitrogen sources in growth medium promoted the enzyme synthesis.
• Broad thermal and pH stability profile indicated its utility in various industrial bioprocesses.
Rights and permissions
About this article
Cite this article
Nawaz, M.A., Bibi, Z., Karim, A. et al. Production of α-1,4-glucosidase from Bacillus licheniformis KIBGE-IB4 by utilizing sweet potato peel. Environ Sci Pollut Res 24, 4058–4066 (2017). https://doi.org/10.1007/s11356-016-8168-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-8168-x