Abstract
An electro/Fe2+/persulfate process has been conducted for toluene removal from surfactant (SDS) flushing solution, and the pseudo-second-order reaction rate constant (k 2 value) of toluene removal has been optimized by a response surface methodology (RSM). The results indicated that in this process, the reaction between persulfate and externally added Fe2+ generates sulfate-free radicals, and at the same time, Fe2+ is electro-regenerated at the cathode by the reduction of Fe3+. RSM based on Box–Behnken design (BBD) has been applied to analyze the experimental variables, of which the concentrations of persulfate and Fe2+ showed a positive effect on the rate constant of toluene removal, whereas the concentration of SDS showed a negative effect. The interactions between pairs of variables proved to be significant, such as between SDS, persulfate, and Fe2+ concentrations. ANOVA results confirmed that the proposed models were accurate and reliable for analysis of the variables of the electro/Fe2+/persulfate process. The shapes of the 3D response surfaces and contour plots showed that the SDS, persulfate, and Fe2+ concentrations substantially affected the k 2 value of toluene removal. The results indicated that increasing persulfate or Fe2+ concentration increased the k 2 value, whereas increasing SDS concentration decreased the k 2 value. The reaction intermediates have been identified by GC-MS, and a plausible degradation pathway for toluene degradation is proposed.
Similar content being viewed by others
Introduction
Benzene, toluene, ethylbenzene, and xylenes (BTEX) is an abbreviation used for four related compounds found in coal tar, crude petroleum, and a wide range of petroleum products. Among them, toluene is one of the most abundantly produced chemicals, with worldwide annual production of 5–10 million tons (ATSDR 2000). The largest source of toluene release is during the production, transport, and use of petroleum, which contains about 5–8 % toluene. Once released into the environment, toluene usually evaporates into the air. Toluene can also dissolve in water, attach to soil particles, and it may be found in surface and groundwater at contaminated sites or in the close vicinity of natural oil, coal, and gas deposits (Li et al. 2013). The main effect of toluene is on the brain and nervous system, with fatigue and drowsiness being the most obvious symptoms (ATSDR 2000), and it can cause neurological damage (ATSDR 2001). In this context, activated persulfate oxidation has emerged as a novel effective remediation technology for the removal of toluene (Do et al. 2011; Huang et al. 2005; Liang et al. 2008, 2009). This system generates sulfate radical (SO4 −), a strong oxidant (E 0 = 2.60 V) that can be thermally or chemically activated by initiators (Shukla et al. 2010; Tan et al. 2013). The activation methods include UV, heat, transition metal ions, alkaline conditions, and persulfate combined with other oxidants. As a natural substrate, ferrous ion (Fe2+) has the advantages of being inexpensive and nontoxic, and it has been widely applied as a catalyst to effectively activate persulfate (Jiang et al. 2013; Kusic et al. 2011; Long et al. 2014a; Xu et al. 2014; Zhao et al. 2014). However, some drawbacks are associated with the Fe2+/persulfate process. For instance, high Fe2+ dosage is required to activate persulfate because Fe2+ is difficult to regenerate after conversion to Fe3+ (Vicente et al. 2011). This results in a large amount of iron sludge. Moreover, the excess Fe2+ could react with SO4 − (Eq. (1)) as a radical scavenger, resulting in reduced efficiency of the process.
In order to solve these problems, an electrochemical method has been applied to the reaction system so that Fe2+ could be regenerated according to Eq. (2) (Wu et al. 2012b):
In this electro/Fe2+/persulfate process, the cathodic reduction reaction enhances the regeneration of Fe2+, which further activates persulfate to generate SO4 − (Wang and Chu 2011; Wu et al. 2012b). As a result, Fe2+ can be continuously regenerated, and the degradation efficiency is improved when an electrochemical potential is applied to the Fe2+/persulfate process. In our previous studies, this process has been successfully applied to the removal of organic pollutants such as Acid Orange 7 (Wu et al. 2012b), bisphenol A (Lin et al. 2013), or clofibric acid (Lin et al. 2014) from water, and even to real wastewater, specifically a landfill leachate (Zhang et al. 2014).
It is generally accepted that surfactant-based treatment provides an effective technology for the remediation of toluene-contaminated soil and groundwater (He et al. 2008; Laha et al. 2009; Lee et al. 2002b; Long et al. 2013). However, most surfactants are too expensive for flushing remediation of hydrophobic organic contaminants, and their residues may be biotoxic in the environment (Ying 2006). Usually, sodium dodecyl sulfate (SDS) is used as a surfactant because it is a food grade material and is easily biodegradable by soil and/or aquatic microorganisms (Lee et al. 2002a). Furthermore, the flushing effluents, containing surfactants and toluene, need to be treated before discharge or reuse. Such pollution necessitates investigations of suitable technologies to control flushing effluents and regenerate the surfactant. In a recent review, SO4 − was proposed as a suitable electrophilic reagent, and when electron-donating groups are present on an aromatic molecule, the rate of the reaction with SO4 − will increase (Long et al. 2014b; Tsitonaki et al. 2010). Moreover, SO4 − is relatively stable in aqueous solution, and thus may be able to disperse more freely (Romero et al. 2010; Vicente et al. 2011). Furthermore, it is generally accepted that SO4 − tends to react selectively via electron transfer (Anipsitakis and Dionysiou 2004; Anipsitakis et al. 2005; Long et al. 2014b; Zhou et al. 2014). Thus, in the flushing effluents, SO4 − may oxidize toluene at a faster rate than it oxidizes straight-chain aliphatic hydrocarbon surfactants. Indeed, selective oxidation of toluene in SDS flushing effluents was observed in our previous studies (Long et al. 2013, 2014a). Therefore, the objective of the present study was to optimize toluene removal for SDS recovery in flushing effluents from the electro/Fe2+/persulfate process using single-factor tests and response surface methodology (RSM). Three reaction parameters (SDS concentration, persulfate concentration, and Fe2+ concentration) were selected as single factors, and the optimal conditions were obtained by the RSM optimization approach. Furthermore, gas chromatography − mass spectrometry (GC-MS) was also applied to determine the intermediates, on the basis of which a degradation pathway of the flushing effluents is proposed.
Materials and methods
Chemicals and reagents
All materials were analytical grade reagents and were used without further purification. Toluene (C6H5CH3) was purchased from Shanghai No. 4 Reagent Factory (China). Sodium persulfate (Na2S2O8), ferrous sulfate (FeSO4 · 7H2O), and sodium dodecyl sulfate (SDS, CH3(CH2)11SO4Na) were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
Apparatus and procedure
Batch experiments were performed in an electrolytic reactor (glass beaker) containing 100 mL of solution. A 5 cm × 11.9 cm plate anode (Ti/RuO2-IrO2) and a plate cathode (stainless steel) of the same dimensions were arranged parallel to one another at a distance of 3 cm. The reactor was immersed in a water bath to keep the temperature constant at 20 °C. Electrolysis was conducted under constant current conditions using a direct current (DC) power supply (Model RYI-3010) from Shenzhen Yizhan Instrument Co., Ltd. (China). A magnetic stirrer (Model 78-1, Hangzhou Instrument Motors Factory, China) ensured mixing of the solution in the reactor. Before each run, a fresh stock solution of toluene was prepared with SDS. The initial concentration (C 0) was fixed at 1 mm, and the initial pH (background value) was around 7.0. When the DC power supply was initiated, persulfate solution was added as a supporting electrolyte, and the current value was kept at 0.5 A to minimize hydrogen and oxygen evolution, and then, Fe2+ solution was introduced into the electrolytic cell. Aliquots of the mixture were sampled and analyzed at preselected time intervals.
Analytical methods
Direct aqueous injection–gas chromatography/flame ionization detection (DAI-GC/FID) was applied to investigate the concentration of toluene in the surfactant solutions (Kubinec et al. 2005; Long et al. 2014a). Samples were quantified using a gas chromatograph (GC-14C, Shimadzu) with the following settings. GC conditions: oven programmed to a constant temperature of 150 °C (12 min), N2 (99.999 % purity) as carrier gas, and SE-54 (30 m × 0.32 mm × 0.50 μm) column. FID conditions: temperature set at 250 °C, H2 flow of 60 mL/min, air flow of 400 mL/min, N2 make-up gas, and make-up flow of 20 mL/min. Injector conditions: injection mode with split inlets (split ratio = 20:1), injection volume of 1 μL, and injector temperature 250 °C.
The intermediate products during the electro/Fe2+/persulfate reaction were detected by headspace–gas chromatography–mass spectrometry (HS-GC-MS) (Esteve-Turrillas et al. 2007). Samples for GC-MS analysis (Shimadzu GCMS-QP2010 Plus, Japan) were prepared as follows. A static headspace auto sampler (Thermo Finnigan model HS 2000, Waltham, MA, USA) equipped with standard glass vials of internal volume 10 mL was employed. Once equilibrium between the matrix and the gaseous phase was reached, a 1-mL vapor sample was injected into the system. An HP-5 MS capillary column (30 m length × 0.25 mm i.d. × 0.25 μm film thickness) was employed for GC separation. The GC equipment was operated in a temperature-programmed mode with an initial temperature of 40 °C held for 2 min, then ramping first to 100 °C at 10 °C min−1 and then further to 200 °C at 20 °C min−1, and finally holding at this temperature for 5 min. The injector and transfer-line temperature was 200 °C. The injector was operated in splitless mode. Helium was used as carrier gas at a flow rate of 5.5 mL/min. The MS-detected scan field was 2–500 m/z, and the ion source temperature was 200 °C.
The concentration of SDS was determined by the methylene blue method (Kenshi 1975). Methylene blue was first added to the supernatant liquid, then the anionic surfactant–cationic dye complex was extracted with chloroform, and the absorption of the chloroform phase was measured at λmax = 655 nm using a Rayleigh UV-9100 spectrophotometer (Rayleigh Co., China). From this figure, the concentration of SDS could be obtained.
Experiment design and data analysis
Box–Behnken design (BBD) was applied to investigate the effects of three independent variables, namely, SDS concentration (X 1), persulfate concentration (X 2), and Fe2+ concentration (X 3), on the response function. The low, middle, and high levels of each variable were coded as −1, 0, and +1, respectively, as illustrated in Table 1. The corresponding actual values were selected based on available data and preliminary experiments. The dependent variable or objective function was the pseudo-second-order removal rate constant (k 2 value). Each independent variable x i was coded as X i according to the following equation (Singh et al. 2012):
where x 0 is the value of x i at the center point and Δx i represents the step change value.
Thus, a total of 15 experiments based on three levels and three variables was required, including three replicates at the center point in order to estimate the pure error sum of squares. All 15 experiments were conducted in random order. Multiple regression analysis is expressed by the second-order polynomial model as follows (Nair and Ahammed 2014):
where Y is the predicted response by the model (k), X i and X j are the independent variables, and β 0, β i, β ii, and β ij are the regression coefficients of the model. The validity of the predicted model was verified by analysis of variance (ANOVA), and the second-order model quality was assessed by the determination coefficient (R 2). The results were analyzed by a Fischer trial and the probability value (with 95 % confidence level). Finally, the optimal values of the tested variables were obtained by analyzing the surface curves.
Results and discussion
Single-factor tests
The toluene removal efficiency depends on several main factors, such as the concentrations of SDS, persulfate, and Fe2+ in the electro/Fe2+/persulfate process. Some preliminary runs were performed to identify suitable single factors. Kinetic analysis illustrated that pseudo-second-order kinetics fitted the experimental data better than pseudo-first-order kinetics. As shown in Tables SM-1 and SM-2, the correlation coefficients (R 2) for pseudo-second-order kinetics ranged from 0.9683 to 0.9924, whereas those for pseudo-first-order kinetics were in the range 0.8035–0.9564.
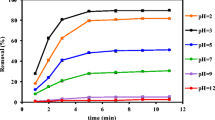
When the SDS concentration was increased from 0.5 to 2 %, the toluene removal rate decreased, as indicated in Fig. 1a. The values of rate constant k 2 were 11.54 × 10−3, 8.28 × 10−3, and 3.02 × 10−3 L mg−1 min−1 at SDS concentrations of 0.5, 1, and 2 %, respectively. The results indicated that there were competing reactions of SO4 − with toluene and SDS. A high concentration of SDS probably prevented the activated radicals from reaching the toluene. Moreover, a higher available Fe2+ concentration improved the decomposition of persulfate to generate SO4 −. As can be seen in Fig. 1b, the toluene removal rate increased when the Fe2+ concentration was increased from 2 to 10 mm. The results indicated that the Fe3+ produced in the reaction could be effectively converted to Fe2+ by electro-reduction, further activating persulfate for SO4 − generation.
In order to further investigate the effect of persulfate on the removal of toluene, a persulfate concentration of 20 mm was used. Under the same reaction conditions, the effect of Fe2+ concentration on the removal of toluene was also investigated by conducting experiments at 0, 2, 5, 10, and 20 mm when the SDS concentration was 2 %. As shown in Fig. 2a, b, the k 2 value increased from 2.33 × 10−4 to 23.2 × 10−4 L mg−1 min−1 as the Fe2+ concentration was increased from 0 to 20 mm. That is to say, increasing the Fe2+ dosage corresponds to a higher available Fe2+ concentration under the electrochemical conditions. Moreover, according to the above results, the k 2 value increased as the persulfate concentration was increased from 20 to 30 mm when the Fe2+ concentration was 5 or 10 mm. This was because persulfate is a source of SO4 − in the system, and so, more of these reactive radicals were generated to degrade toluene at higher persulfate concentrations. The k 2 value was 2.33 × 10−4 L mg−1 min−1 in the absence of Fe2+. This result indicated that persulfate could generate SO4 − by an electron-transfer reaction (Eq. (5)) (Lin et al. 2013; Wu et al. 2012b).
Response surface evaluation
The full factorial analysis with three factors at three levels is presented in Table 2, along with the k 2 values for the respective runs. Based on the data in Table 2, the main effects plot and the interaction plots for k 2 values at 30 min were developed. The main effects plot indicated that the effect of the Fe2+ or persulfate concentration on the k 2 value was positive, whereas that of the SDS concentration was negative (Fig. SM-1). Thus, a high level (+1) of Fe2+ or persulfate concentration, but a low level (−1) of SDS concentration would lead to a higher k 2 value (Wu et al. 2012a; Zhang et al. 2009, 2010).
The pretreatment conditions were optimized by employing a Box–Behnken design (BBD) and a polynomial equation describing the k 2 value over 30 min as a simultaneous function of SDS concentration (X 1), persulfate concentration (X 2), and Fe2+ concentration (X 3), as shown in Eq. (6):
The effects of two-factor interactions were investigated with one factor fixed at a high (+1) or low (−1) level while the other was varied (Gong et al. 2010; Rastegar et al. 2011). Figure SM-2 shows interaction plots, which indicate whether or not there was an interaction between the factors. Here, the interaction between each pair of variables, namely, SDS, persulfate, and Fe2+ concentrations, was significant, as indicated by the curves crossing. That is to say, a change in one variable would affect the other. This was also confirmed by the high probability value ((Prob > F) > 0.1) obtained through analysis of variance (ANOVA).
The ANOVA results in Table 3 were used to assess the significance and adequacy of the model. The value of (Prob > F) was used to determine the significance of each model term. Corresponding model terms are more significant with decreasing value of (Prob > F), whereas a value over 0.1 implies insignificance (Zhang et al. 2011a). According to the selected confidence level ((Prob > F) > 0.1), the insignificant model term (X 2 2) was removed from the RSM models; Eq. (6) could then be simplified to Eq. (7), and the ultimate RSM models, in terms of the coded factors, were determined to calculate the rate constants.
The very low (Prob > F) values (<0.0001) and large absolute F value of 111.82 imply that the model is significant for the 30-min rate constants (Zhang et al. 2011b; Long et al. 2013; Sekaran et al. 2014). The determination coefficient (R 2) provides a measure of how much variability in the response values can be attributed to the experimental variables and their interactions (Long et al. 2014a; Nair and Ahammed 2014). The determination coefficient (R 2 = 0.9951) of the removal efficiency by ANOVA indicated that only 0.49 % of the total variation could not be explained by the quadratic regression model. The value of the adjusted determination coefficient (Adj. R 2) corrects the determination coefficient for the sample size and the number of terms and does not always increase when adding variables (Wu et al. 2012a). In this experiment, the Adj. R 2 value (0.9885) was very close to the corresponding R 2 value. This further confirmed that the model was highly significant. Moreover, the nonsignificant lack-of-fit (p value = 0.0319) suggests that the obtained experimental data fit well with the model (Ferreira et al. 2007). Furthermore, the coefficient of variation (CV) describes the extent to which the data were dispersed, with lower values giving better reproducibility (Ahmad et al. 2005; Sekaran et al. 2014). The present CV value (CV = 9.55 %) indicated that the variation in the mean value was satisfactory, being within the acceptable range of 0.5–13.5 % (Long et al. 2013, 2014a; Sekaran et al. 2014). Finally, adequate precision is used to measure the signal-to-noise ratio. A value of adequate precision greater than 4 is desirable. Therefore, the adequate precision value of 40.379 indicated acceptable signals for the models to be used to navigate the design space (Kayan and Gözmen 2012; Wu et al. 2012a).
The adequacy of the model, which is an important part of the data analysis process, was also evaluated. Poor or misleading results would be obtained if the model were not an adequate fit. The normal probability and studentized residual plots indicate whether the studentized residuals follow a normal distribution. As is evident from Fig. SM-3, there was no apparent problem with normality, and no need for transformation of the response because the normal residuals fell along a straight line. Figure SM-4 shows the studentized residuals plotted against predicted values, and the residuals appear to show a random scatter. This suggests that the equality of variance did not seem to be violated. Furthermore, the similarity between the predicted (from Eq. (7)) and actual experimental results presented in Fig. 3 reflects the accuracy and applicability of the Box–Behnken model as a powerful method for process optimization.
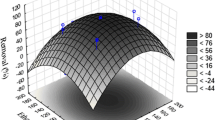
3D response surface plots for the k 2 value were evaluated using Eq. (7) and are illustrated in Fig. 4. To delineate the interactive effects of independent variables on the responses, one variable was kept constant while the other two were varied. The shapes of the response surfaces and contour plots indicate the nature and extent of the interaction between the different factors (Bremner et al. 2009). As can be seen in Fig. 4, optimal persulfate and Fe2+ concentrations for maximizing the k 2 value were seen when these variables were set at high levels (+1). This may have been because increases in persulfate and Fe2+ concentration initially enhanced the SO4 − generation. Moreover, increasing persulfate concentration would not lead to consumption reaction between persulfate and SO4 − (reaction (8)) (Wu et al. 2012b) because excess persulfate could be reduced by electrons (reaction (5)) to produce SO4 −. Furthermore, even though increasing Fe2+ concentration would lead to its consumption reaction with SO4 − (reaction (1)), which would compete with SO4 − generation, the generated Fe3+ could be reduced with electrons to produce Fe2+ for the continuous activation of persulfate. Increasing SDS concentration seemed to have a negative effect on toluene removal rate. The results were indicative of competing reactions of SO4 − with toluene and SDS. A high concentration of SDS prevented the formation of activated radicals for the degradation of toluene, in accordance with the results from the single-factor tests.
Response surface showing toluene degradation rate constants as a function of two independent variables (other variables were held at their respective center levels). a SDS concentration (X 1) and persulfate concentration (X 2). b SDS concentration (X 1) and Fe2+ concentration (X 3). c Persulfate concentration (X 2) and Fe2+ concentration (X 3)
Selective oxidation of toluene for surfactant recovery
The SO4 − generated from the electro/Fe2+/persulfate process was applied in the selective oxidation of toluene for SDS recovery from flushing effluents. Compared with the Fe2+/persulfate process, as shown in Fig. SM-5, higher toluene removal efficiency was obtained by the electro/Fe2+/persulfate process when the reaction conditions were based on the optimization approach of previous research (Long et al. 2014a). The results further confirmed that the electrochemical method could be suitably applied for the treatment of flushing effluents for surfactant recovery. Moreover, variations in the toluene removal efficiency from the SDS solutions were investigated by performing the electro/Fe2+/persulfate process with different SDS concentrations of 0.5, 1, and 2 % (w/v). Under the RSM optimization conditions (lower SDS concentration of 0.5 % (coded value −1), higher persulfate concentration of 30 mm (coded value +1), and Fe2+ concentration of 20 mm (coded value +1)), toluene was completely removed after 30 min (data not shown). However, only a little SDS was removed (data not shown) in the electro/Fe2+/persulfate system when its concentration was 0.5, 1, or 2 %, consistent with the results of previous research (Long et al. 2014a). These results indicated that there were competing reactions of the SO4 − with toluene and SDS. Therefore, a high concentration of SDS was likely to give a lower toluene removal efficiency. Importantly, SO4 − has been proved to be potentially effective for selectively degrading toluene while leaving most of the surfactant intact for recovery.
Degradation pathway of toluene
Direct detection of the degradation intermediates was essential for explaining and understanding the mechanism of the selective oxidation of toluene in the surfactant flushing solution. To further identify the intermediate products, GC-MS analysis was employed. The intermediate products identified after the electro/Fe2+/persulfate treatment are shown in Table 4. Based on these results and those of previous studies (Long et al. 2014a; Nomiya et al. 2001; Van Durme et al. 2007; Wang et al. 2009), a plausible degradation pathway is proposed (Fig. 5). First, SO4 − removes an electron from toluene (C) to produce an organic radical cation for the next oxidation process. However, some toluene (C) remains intact in the electro/Fe2+/persulfate process. Intermediates benzene (B) and benzaldehyde (F) showed that toluene reacts with SO4 − by a pathway involving hydrogen atom abstraction from the methyl group, leading to a benzyl radical. Moreover, intermediates butanal (A), hexanal (D), and heptanal (E) showed that SO4 − was directly added to the aromatic ring of toluene. Further oxidation reactions lead to degradation of these intermediates to smaller molecules. Finally, the intermediate products were mineralized to CO2, H2O, and inorganic ions. Additionally, the intermediates octanal (G), undecane (H), nonanal (I), and undecanal (J) indicated that a small portion of the surfactant SDS may have been attacked by radicals such as SO4 −.
Conclusions
This study has shown that the electro/Fe2+/persulfate process is effective for removing toluene from SDS solution, with Fe3+ being concomitantly reduced to Fe2+. Single-factor tests have indicated that SO4 − is potentially effective for selectively degrading toluene in surfactant flushing effluents. It has been further proved that high SDS concentration disfavors selective oxidation by SO4 −, the rate constants decreasing with increasing SDS concentration. However, higher persulfate and Fe2+ concentrations favor toluene removal from the surfactant solution. The process has been optimized by an RSM approach. The results indicated that the three variables of SDS concentration, persulfate concentration, and Fe2+ concentration had significant effects on the removal of toluene. The main intermediates were separated and identified by GC-MS, and a plausible degradation pathway of toluene has been proposed.
References
Ahmad AL, Ismail S, Bhatia S (2005) Optimization of coagulation-flocculation process for palm oil mill effluent using response surface methodology. Environ Sci Technol 39:2828–2834
Anipsitakis GP, Dionysiou DD (2004) Radical generation by the interaction of transition metals with common oxidants. Environ Sci Technol 38:3705–3712
Anipsitakis GP, Dionysiou DD, Gonzalez MA (2005) Cobalt-mediated activation of peroxymonosulfate and sulfate radical attack on phenolic compounds. Implications of chloride ions. Environ Sci Technol 40:1000–1007
ATSDR (2000) Toxicological profile for toluene. U.S. Department of Health and Human Services, Public Health Service, Atlanta
ATSDR (2001) Interaction profile for benzene, toluene, ethylbenzene and xylenes (BTEX). U.S. Department of Health and Human Services, Public Health Service, Atlanta
Bremner DH, Molina R, Martínez F, Melero JA, Segura Y (2009) Degradation of phenolic aqueous solutions by high frequency sono-Fenton systems (US-Fe2O3/SBA-15-H2O2). Appl Catal B Environ 90:380–388
Do SH, Kwon YJ, Kong SH (2011) Feasibility study on an oxidant-injected permeable reactive barrier to treat BTEX contamination: Adsorptive and catalytic characteristics of waste-reclaimed adsorbent. J Hazard Mater 191:19–25
Esteve-Turrillas FA, Armenta S, Garrigues S, Pastor A, de la Guardia M (2007) Headspace-mass spectrometry determination of benzene, toluene and the mixture of ethylbenzene and xylene isomers in soil samples using chemometrics. Anal Chim Acta 587:89–96
Ferreira SLC, Bruns RE, Ferreira HS, Matos GD, David JM, Brandão GC, da Silva EGP, Portugal LA, dos Reis PS, Souza AS, dos Santos WNL (2007) Box-Behnken design: An alternative for the optimization of analytical methods. Anal Chim Acta 597:179–186
Gong YH, Zhang H, Li YL, Xiang LJ, Royer S, Valange S, Barrault J (2010) Evaluation of heterogeneous photo-Fenton oxidation of Orange II using response surface methodology. Water Sci Technol 62:1320–1326
He L, Huang GH, Lu HW, Zeng GM (2008) Optimization of surfactant-enhanced aquifer remediation for a laboratory BTEX system under parameter uncertainty. Environ Sci Technol 42:2009–2014
Huang KC, Zhao ZQ, Hoag GE, Dahmani A, Block PA (2005) Degradation of volatile organic compounds with thermally activated persulfate oxidation. Chemosphere 61:551–560
Jiang XX, Wu YL, Wang P, Li HJ, Dong WB (2013) Degradation of bisphenol A in aqueous solution by persulfate activated with ferrous ion. Environ Sci Pollut Res 20:4947–4953
Kayan B, Gözmen B (2012) Degradation of Acid Red 274 using H2O2 in subcritical water: Application of response surface methodology. J Hazard Mater 201–202:100–106
Kenshi H (1975) A rapid determination of sodium dodecyl sulfate with methylene blue. Anal Biochem 67:503–506
Kubinec R, Adamuščin J, Jurdáková H, Foltin M, Ostrovský I, Kraus A, Soják L (2005) Gas chromatographic determination of benzene, toluene, ethylbenzene and xylenes using flame ionization detector in water samples with direct aqueous injection up to 250 μl. J Chromatogr A 1084:90–94
Kusic H, Peternel I, Ukic S, Koprivanac N, Bolanca T, Papic S, Bozic AL (2011) Modeling of iron activated persulfate oxidation treating reactive azo dye in water matrix. Chem Eng J 172:109–121
Laha S, Tansel B, Ussawarujikulchai A (2009) Surfactant-soil interactions during surfactant-amended remediation of contaminated soils by hydrophobic organic compounds: A review. J Environ Manage 90:95–100
Lee DH, Cody RD, Kim DJ (2002a) Surfactant recycling by solvent extraction in surfactant-aided remediation. Sep Purif Technol 27:77–82
Lee DH, Cody RD, Kim DJ, Choi S (2002b) Effect of soil texture on surfactant-based remediation of hydrophobic organic-contaminated soil. Environ Int 27:681–688
Li XF, Zhou QX, Luo Y, Yang G, Zhou T (2013) Joint action and lethal levels of toluene, ethylbenzene, and xylene on midge (Chironomus plumosus) larvae. Environ Sci Pollut Res 20:957–966
Liang CJ, Huang CF, Chen YJ (2008) Potential for activated persulfate degradation of BTEX contamination. Water Res 42:4091–4100
Liang CJ, Chen YJ, Chang KJ (2009) Evaluation of persulfate oxidative wet scrubber for removing BTEX gases. J Hazard Mater 164:571–579
Lin H, Wu J, Zhang H (2013) Degradation of bisphenol A in aqueous solution by a novel electro/Fe3+/peroxydisulfate process. Sep Purif Technol 117:18–23
Lin H, Wu J, Zhang H (2014) Degradation of clofibric acid in aqueous solution by an EC/Fe3+/PMS process. Chem Eng J 244:514–521
Long AH, Zhang H, Lei Y (2013) Surfactant flushing remediation of toluene contaminated soil: Optimization with response surface methodology and surfactant recovery by selective oxidation with sulfate radicals. Sep Purif Technol 118:612–619
Long AH, Lei Y, Zhang H (2014a) Degradation of toluene by a selective ferrous ion activated persulfate oxidation process. Ind Eng Chem Res 53:1033–1039
Long AH, Lei Y, Zhang H (2014b) In situ chemical oxidation of organic contaminated soil and groundwater using activated persulfate process. Prog Chem 26:898–908
Nair AT, Ahammed MM (2014) Coagulant recovery from water treatment plant sludge and reuse in post-treatment of UASB reactor effluent treating municipal wastewater. Environ Sci Pollut Res 21:10407–10418
Nomiya K, Hashino K, Nemoto Y, Watanabe M (2001) Oxidation of toluene and nitrobenzene with 30 % aqueous hydrogen peroxide catalyzed by vanadium(V)-substituted polyoxometalates. J Mol Catal A Chem 176:79–86
Rastegar SO, Mousavi SM, Shojaosadati SA, Sheibani S (2011) Optimization of petroleum refinery effluent treatment in a UASB reactor using response surface methodology. J Hazard Mater 197:26–32
Romero A, Santos A, Vicente F, González C (2010) Diuron abatement using activated persulphate: Effect of pH, Fe(II) and oxidant dosage. Chem Eng J 162:257–265
Sekaran G, Karthikeyan S, Boopathy R, Maharaja P, Gupta VK, Anandan C (2014) Response surface modeling for optimization heterocatalytic Fenton oxidation of persistence organic pollution in high total dissolved solid containing wastewater. Environ Sci Pollut Res 21:1489–1502
Shukla PR, Wang SB, Ang HM, Tadé MO (2010) Photocatalytic oxidation of phenolic compounds using zinc oxide and sulphate radicals under artificial solar light. Sep Purif Technol 70:338–344
Singh KP, Singh AK, Gupta S, Rai P (2012) Modeling and optimization of reductive degradation of chloramphenicol in aqueous solution by zero-valent bimetallic nanoparticles. Environ Sci Pollut Res 19:2063–2078
Tan C, Gao N, Deng Y, Rong W, Zhou S, Lu N (2013) Degradation of antipyrine by heat activated persulfate. Sep Purif Technol 109:122–128
Tsitonaki A, Petri B, Crimi M, Mosbaek H, Siegrist RL, Bjerg PL (2010) In situ chemical oxidation of contaminated soil and groundwater using persulfate: A review. Crit Rev Env Sci Technol 40:55–91
Van Durme J, Dewulf J, Sysmans W, Leys C, Van Langenhove H (2007) Abatement and degradation pathways of toluene in indoor air by positive corona discharge. Chemosphere 68:1821–1829
Vicente F, Santos A, Romero A, Rodriguez S (2011) Kinetic study of diuron oxidation and mineralization by persulphate: Effects of temperature, oxidant concentration and iron dosage method. Chem Eng J 170:127–135
Wang YR, Chu W (2011) Degradation of 2,4,5-trichlorophenoxyacetic acid by a novel Electro-Fe(II)/Oxone process using iron sheet as the sacrificial anode. Water Res 45:3883–3889
Wang X, Wu J, Zhao M, Lv Y, Li G, Hu C (2009) Partial oxidation of toluene in CH3COOH by H2O2 in the presence of VO (acac)2 catalyst. J Phy Chem C 113:14270–14278
Wu J, Zhang H, Oturan N, Wang Y, Chen L, Oturan MA (2012a) Application of response surface methodology to the removal of the antibiotic tetracycline by electrochemical process using carbon-felt cathode and DSA (Ti/RuO2–IrO2) anode. Chemosphere 87:614–620
Wu J, Zhang H, Qiu JJ (2012b) Degradation of Acid Orange 7 in aqueous solution by a novel electro/Fe2+/peroxydisulfate process. J Hazard Mater 215–216:138–145
Xu HB, Zhao DY, Li YJ, Liu PY, Dong CX (2014) Enhanced degradation of ortho-nitrochlorobenzene by the combined system of zero-valent iron reduction and persulfate oxidation in soils. Environ Sci Pollut Res 21:5132–5140
Ying GG (2006) Fate, behavior and effects of surfactants and their degradation products in the environment. Environ Int 32:417–431
Zhang H, Choi HJ, Canazo P, Huang CP (2009) Multivariate approach to the Fenton process for the treatment of landfill leachate. J Hazard Mater 161:1306–1312
Zhang H, Li YL, Wu XG, Zhang YJ, Zhang DB (2010) Application of response surface methodology to the treatment landfill leachate in a three-dimensional electrochemical reactor. Waste Manage 30:2096–2102
Zhang H, Li YL, Zhong X, Ran XN (2011a) Application of experimental design methodology to the decolorization of Orange II using low iron concentration of photoelectro-Fenton process. Water Sci Technol 63:1373–1380
Zhang H, Ran XN, Wu XG, Zhang DB (2011b) Evaluation of electro-oxidation of biologically treated landfill leachate using response surface methodology. J Hazard Mater 188:261–268
Zhang H, Wang Z, Liu CC, Guo YZ, Shan N, Meng CX, Sun LY (2014) Removal of COD from landfill leachate by an electro/Fe2+/peroxydisulfate process. Chem Eng J 250:76–82
Zhao L, Hou H, Fujii A, Hosomi M, Li FS (2014) Degradation of 1,4-dioxane in water with heat- and Fe2+-activated persulfate oxidation. Environ Sci Pollut Res 21:7457–7465
Zhou DN, Zhang H, Chen L (2014) Sulfur-replaced Fenton systems: can sulfate radical substitute hydroxyl radical for advanced oxidation technologies? J Chem Technol Biotechnol. doi:10.1002/jctb.4525
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No 20977069). LONG A.H. would like to acknowledge the Ph.D. Research Startup Foundation of Jiangxi Science and Technology Normal University (Grant No. 3000990115).
Conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Angeles Blanco
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 476 KB)
Rights and permissions
About this article
Cite this article
Long, A., Zhang, H. Selective oxidative degradation of toluene for the recovery of surfactant by an electro/Fe2+/persulfate process. Environ Sci Pollut Res 22, 11606–11616 (2015). https://doi.org/10.1007/s11356-015-4406-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4406-x