Abstract
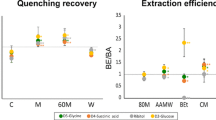
A highly selective, sensitive and nonradioactive analytical method for identification and quantification of intracellular metabolites involved in isoprenoid pathway has been developed by means of gas chromatography-selected ion-monitoring mass spectrometry (GC-SIM-MS). These metabolites are classified into two groups: sterols (squalene, ergosterol, lanosterol) and phosphorylated compounds (geranyl diphosphate, farnesyl pyrophosphate, geranylgeranyl pyrophosphate) based on their physicochemical properties. To quantify both groups in a single analytical run, GPP, FPP and GGPP were cleaved to the parent alcohols, geraniol, farnesol, geranylgeraniol by pyrophosphatase followed by alkaline phosphatase before extraction, separation and detection. This study evaluated several extraction procedures and determined the effects of the type of extraction solvent, times used for extraction. Under optimized GC/EI-MS conditions, six compounds were separated with high efficiency in the selected-ion monitoring (SIM) mode. Linearity of the method was good with correlation coefficients (r 2) in the range of 0.9953–0.9999 and detection limits were 1.53–151.88 ng/ml. The intra-day and inter-day precision of the method, as RSD, were less than 5.31 and 6.04%, respectively. The accuracy of six compounds varied between 87.7 and 110.8%. This assay was successfully applied to the determination of six major metabolites in the pathway for isoprenoid biosynthesis in S. cerevisiae and is sensitive to detect changes following genetic modification. By isolating statistically significant differences among metabolite levels from four biological conditions, we observed discriminatory metabolic features that hinted that the role of erg9 and coq1gene was involved in isoprenoid pathway. Integrating this analytical approach with statistical strategies, we can determine the influence of erg9 and coq1gene on isoprenoid levels of S. cerevisiae, thus leading to improved understanding of the pathway in a multitude of biological systems.





Similar content being viewed by others
References
Asadollahi, M. A., Maury, J., Moller, K., Nielsen, K. F., Schalk, M., Clark, A., et al. (2008). Production of plant sesquiterpenes in Saccharomyces cerevisiae: Effect of ERG9 repression on sesquiterpene biosynthesis. Biotechnology and Bioengineering, 99, 666–677.
Beyer, P., Kreuz, K., & Kleinig, H. (1985). Separation of mevalonate phosphates and isopentenyl pyrophosphate by thin-layer chromatography and of short-chain prenyl phosphates by ion-pair chromatography on a high-performance liquid chromatography column. Methods in Enzymology, 111, 248–252.
Bruenger, E., & Rilling, H. C. (1988). Determination of isopentenyl diphosphate and farnesyl diphosphate in tissue samples with a comment on secondary regulation of polyisoprenoid biosynthesis. Analytical Biochemistry, 173, 321–327.
Daum, G., Lees, N. D., Bard, M., & Dickson, R. (1998). Biochemistry, cell biology and molecular biology of lipids of Saccharomyces cerevisiae. Yeast, 14, 1471–1510.
Dutta, P. C., & Normén, L. (1998). Capillary column gas-liquid chromatographic separation of [Delta]5-unsaturated and saturated phytosterols. Journal of Chromatography A, 816, 177–184.
Ellington, J. J., Schlotzhauer, P. F., & Schepartz, A. I. (1977). Quantitation of tobacco lipids. Journal of Chromatographic Science, 15, 295–300.
Grob, K., Lanfranchi, M., & Mariani, C. (1989). Determination of free and esterified sterols and of wax esters in oils and fats by coupled liquid chromatography-gas chromatography. Journal of Chromatography A, 471, 397–405.
Henneman, L., van Cruchten, A. G., Denis, S. W., Amolins, M. W., Placzek, A. T., Gibbs, R. A., et al. (2008). Detection of nonsterol isoprenoids by HPLC-MS/MS. Analytical Biochemistry, 383, 18–24.
Hooff, G. P., Volmer, D. A., Wood, W. G., Muller, W. E., & Eckert, G. P. (2008). Isoprenoid quantitation in human brain tissue: A validated HPLC-fluorescence detection method for endogenous farnesyl- (FPP) and geranylgeranylpyrophosphate (GGPP). Analytical and Bioanalytical Chemistry, 392, 673–680.
Jemal, M., Schuster, A., & Whigan, D. B. (2003). Liquid chromatography/tandem mass spectrometry methods for quantitation of mevalonic acid in human plasma and urine: Method validation, demonstration of using a surrogate analyte, and demonstration of unacceptable matrix effect in spite of use of a stable isotope analog internal standard. Rapid Communications in Mass Spectrometry, 17, 1723–1734.
Lechner, M., Reiter, B., & Lorbeer, E. (1999). Determination of tocopherols and sterols in vegetable oils by solid-phase extraction and subsequent capillary gas chromatographic analysis. Journal of Chromatography A, 857, 231–238.
Lees, N. D., Bard, M., & Kirsch, D. R. (1999). Biochemistry and molecular biology of sterol synthesis in Saccharomyces cerevisiae. Critical Reviews in Biochemistry and Molecular Biology, 34, 33–47.
Li, T. S. C., Beveridge, T. H. J., & Drover, J. C. G. (2007). Phytosterol content of sea buckthorn (Hippophae rhamnoides L.) seed oil: Extraction and identification. Food Chemistry, 101, 1633–1639.
Phillips, K. M., Ruggio, D. M., & Bailey, J. A. (1999). Precise quantitative determination of phytosterols, stanols, and cholesterol metabolites in human serum by capillary gas-liquid chromatography. Journal of Chromatography B: Biomedical Sciences and Applications, 732, 17–29.
Richards, J. B., & Hemming, F. W. (1972). Dolichols, ubiquinones, geranylgeraniol and farnesol as the major metabolites of mevalonate in Phytophthora cactorum. Biochemical Journal, 128, 1345–1352.
Saini, G. S., Wani, T. A., Gautam, A., Varshney, B., Ahmed, T., Rajan, K. S., et al. (2006). Validation of the LC-MS/MS method for the quantification of mevalonic acid in human plasma and determination of the matrix effect. Journal of Lipid Research, 47, 2340–2345.
Scoppola, A., Maher, V. M., Thompson, G. R., Rendell, N. B., & Taylor, G. W. (1991). Quantitation of plasma mevalonic acid using gas chromatography-electron capture mass spectrometry. Journal of Lipid Research, 32, 1057–1060.
Severson, R. F., Ellington, J. J., Arrendale, R. F., & Snook, M. E. (1978). Quantitative gas chromatographic method for the analysis of aliphatic hydrocarbons, terpenes, fatty alcohols, fatty acids and sterols in tobacco. Journal of Chromatography A, 160, 155–168.
Shiba, Y., Paradise, E. M., Kirby, J., Ro, D. K., & Keasling, J. D. (2007). Engineering of the pyruvate dehydrogenase bypass in Saccharomyces cerevisiae for high-level production of isoprenoids. Metabolic Engineering, 9, 160–168.
Siavoshian, S., Simoneau, C., Maugeais, P., Marks, L., Rodary, L., Gardette, J., et al. (1995). Measurement of mevalonic acid in human urine by bench top gas chromatography-mass spectrometry. Clinica Chimica Acta, 243, 129–136.
Song, L. (2003). Detection of farnesyl diphosphate accumulation in yeast ERG9 mutants. Analytical Biochemistry, 317, 180–185.
Tong, H., Holstein, S. A., & Hohl, R. J. (2005). Simultaneous determination of farnesyl and geranylgeranyl pyrophosphate levels in cultured cells. Analytical Biochemistry, 336, 51–59.
Tong, H., Wiemer, A. J., Neighbors, J. D., & Hohl, R. J. (2008). Quantitative determination of farnesyl and geranylgeranyl diphosphate levels in mammalian tissue. Analytical Biochemistry, 378, 138–143.
Vallon, T., Ghanegaonkar, S., Vielhauer, O., Muller, A., Albermann, C., Sprenger, G., et al. (2008). Quantitative analysis of isoprenoid diphosphate intermediates in recombinant and wild-type Escherichia coli strains. Applied Microbiology and Biotechnology, 81, 175–182.
Veen, M., & Lang, C. (2004). Production of lipid compounds in the yeast Saccharomyces cerevisiae. Applied Microbiology and Biotechnology, 63, 635–646.
Verwaal, R., Wang, J., Meijnen, J. P., Visser, H., Sandmann, G., van den Berg, J. A., et al. (2007). High-level production of beta-carotene in Saccharomyces cerevisiae by successive transformation with carotenogenic genes from Xanthophyllomyces dendrorhous. Applied and Environmental Microbiology, 73, 4342–4350.
Woollen, B. H., Holme, P. C., Northway, W. J., & Martin, P. D. (2001). Determination of mevalonic acid in human urine as mevalonic acid lactone by gas chromatography-mass spectrometry. Journal of Chromatography B: Biomedical Sciences and Applications, 760, 179–184.
Acknowledgments
This research was funded by Shanghai Science and Technology Committee (No. 054319936) and the National Natural Science Foundation of China (No. 20702062)
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Huang, B., Zeng, H., Dong, L. et al. Metabolite target analysis of isoprenoid pathway in Saccharomyces cerevisiae in response to genetic modification by GC-SIM-MS coupled with chemometrics. Metabolomics 7, 134–146 (2011). https://doi.org/10.1007/s11306-010-0240-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11306-010-0240-9